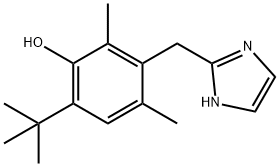
2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
- CAS NO.:526-36-3
- Empirical Formula: C16H24N2
- Molecular Weight: 244.38
- MDL number: MFCD00242956
- EINECS: 208-390-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:34:03
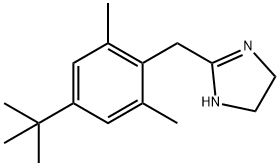
What is 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE?
Absorption
No information is available on xylometazoline pharmacokinetics.
Toxicity
The oral LD50 is 230 mg/kg in rats and 75 mg/kg in mice. The subcutaneous LD50 is 90 mg/kg in rats and 53 mg/kg in mice. The intraperitoneal LD50 is 43 mg/kg in rats.
Xylometazoline poisoning is documented in three pediatric patients who were exposed to a drug concentration 40 times above the adequate dosage for children due to a compounding error: these patients experienced bradypnea and sinus bradycardia with supraventricular extrasystoles and were managed with fluid management.
The Uses of 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
Adrenergic (vasoconstrictor).
The Uses of 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
Xylometazoline is used for rhinitis, laryngitis, sinusitis, inflammation of antrum of Highmore, and allergic illnesses of the nasal cavity and throat.
Background
Xylometazoline is an imidazoline derivative with sympathomimetic and nasal decongestant activity. Xylometazoline works by binding to alpha (α)-adrenergic receptors to cause vasoconstriction of nasal blood vessels.
Xylometazoline is available in over-the-counter (OTC) nasal sprays or drops to temporarily relieve nasal congestion due to cold, hay fever or other respiratory allergies. In some countries, it is available as combination products with ipratropium, domiphen, or dexpanthenol.
Indications
Xylometazoline is indicated for the temporary relief of nasal congestion due to cold, hay fever or other respiratory allergies.
Definition
ChEBI: Xylometazoline is an alkylbenzene.
brand name
Neo-Synephrine II (Sterling Winthrop); Otrivin Hydrochloride (Ciba-Geigy).
Pharmacokinetics
Xylometazoline is a sympathomimetic agent that causes vasoconstriction of the nasal mucosa. In one study comprising subjects with nasal congestion associated with the common cold, the median time of onset of subjective relief of nasal congestion was about 1.7 minutes and the time of subjective peak relief of nasal congestion was 30 minutes. Previous studies reported rebound swelling, rebound nasal congestion, rhinitis medicamentosa, and shorter duration of decongestant effect from the long-term use of xylometazoline in healthy volunteers, suggesting that the drug is most effective if used temporarily.
An early in vitro study demonstrated xylometazoline to exert anti-oxidant actions, where it inhibited microsomal lipid peroxidation and mediated hydroxyl radical scavenging activity. This suggests that xylometazoline has a beneficial effect against oxidants, which play a role in tissue damage in inflammation.
Synthesis
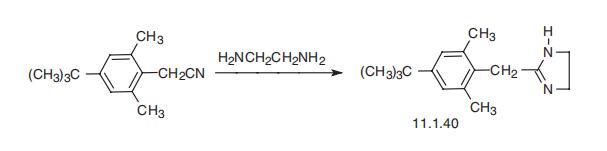
Xylometazoline, 2-(4-tert-butyl-2,6-dimethylbenzyl)-2-imidazoline (11.1.40), is also synthesized in a single reaction by cyclocondensation of 4-tert-butyl-2,6- dimethylbenzylcyanide with ethylendiamine [43,44].
Metabolism
No information is available on xylometazoline pharmacokinetics.
Properties of 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
| Melting point: | 131-133° |
| Boiling point: | 394.2±21.0 °C(Predicted) |
| Density | 1.00±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 49 mg/mL (200.51 mM);Ethanol: 49 mg/mL (200.51 mM) |
| pka | pKa 10.6±0.1(H2O
t = 22.0) (Uncertain) |
| Water Solubility | Water: Insoluble |
| CAS DataBase Reference | 526-36-3 |
Safety information for 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
Computed Descriptors for 2-(4-TERT-BUTYL-2,6-DIMETHYL-BENZYL)-4,5-DIHYDRO-1H-IMIDAZOLE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
