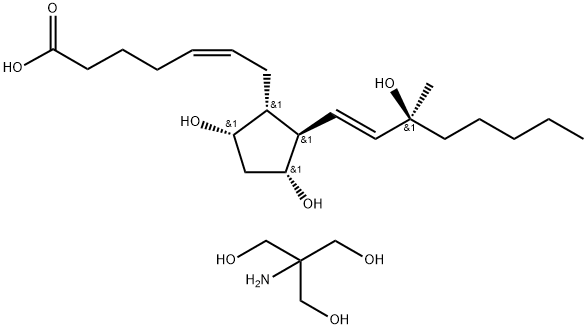
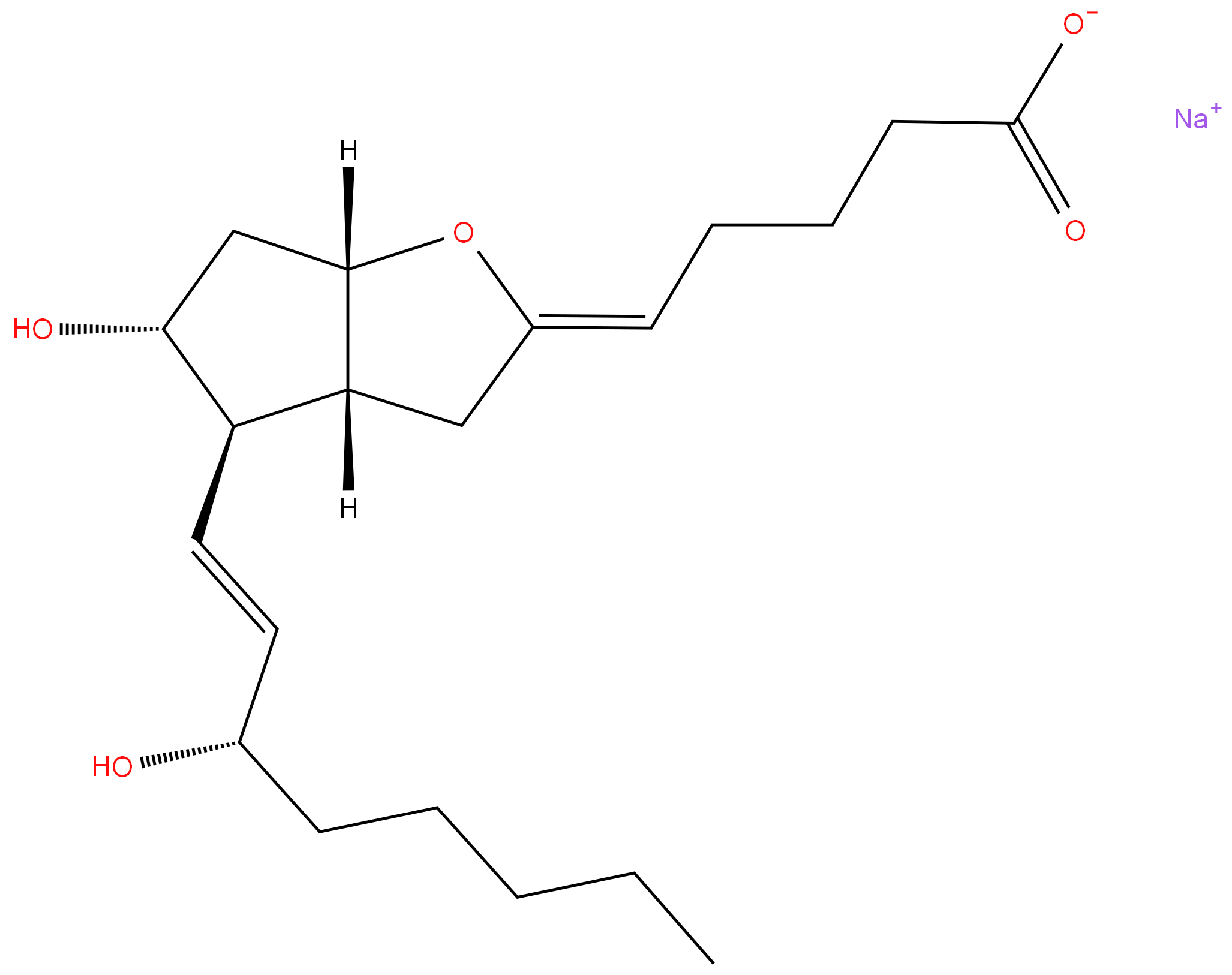
Epoprostenol
- CAS NO.:35121-78-9
- Empirical Formula: C20H32O5
- Molecular Weight: 352.47
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
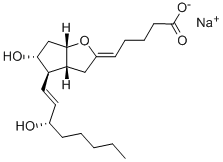
What is Epoprostenol?
Toxicity
Symptoms of overdose are extensions of its dose-limiting pharmacologic effects and include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Most events were self-limiting and resolved with reduction or withholding of epoprostenol. Single intravenous doses at 10 and 50 mg/kg (2703 and 27,027 times the recommended acute phase human dose based on body surface area) were lethal to mice and rats, respectively. Symptoms of acute toxicity were hypoactivity, ataxia, loss of righting reflex, deep slow breathing, and hypothermia.
Originator
Prostacyclin,ZYF Pharm Chemical
The Uses of Epoprostenol
Inhibitor (platelet). [Names previously used: Prostacyclin, PGI2, Prostagland in I2, Prostaglandin X, PGX].
Background
A prostaglandin that is a powerful vasodilator and inhibits platelet aggregation. It is biosynthesized enzymatically from prostaglandin endoperoxides in human vascular tissue. The sodium salt has been also used to treat primary pulmonary hypertension.
Indications
For the long-term intravenous treatment of primary pulmonary hypertension and pulmonary hypertension associated with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy.
Definition
ChEBI: Prostaglandin I2 is a prostaglandins I. It has a role as a mouse metabolite. It is a conjugate acid of a prostaglandin I2(1-).
Manufacturing Process
Preparation of prostacyclin:
Pig aortas were stripped of adventitia, snap frozen in liquid nitrogen, crushed
into a fine powder, resuspended in 0.05 M Tris buffer (pH 7.5) (1:4, w:v) andhomogenised at high speed in a Polytron (KIMENATIC, LUCERNE,
SWITZERLAND) homogenizer. The homogenate was centrifuged for 15 min
and the resulting supernatant centrifuged again for 5 min. The pellet was
discarded, while the pellet obtained after centrifugation of the supernatant
was resuspended in deionized water and lyophilized. An average yield of 150
mg of aortic microsomal powder (51% protein) per 100 g of aortic tissue was
obtained.
brand name
Flolan (GlaxoSmithKline).
Therapeutic Function
Platelet aggregation inhibitor, Antimetastatic
Pharmacokinetics
Epoprostenol has two major pharmacological actions: (1) direct vasodilation of pulmonary and systemic arterial vascular beds, and (2) inhibition of platelet aggregation. In animals, the vasodilatory effects reduce right and left ventricular afterload and increase cardiac output and stroke volume. The effect of epoprostenol on heart rate in animals varies with dose. At low doses, there is vagally mediated brudycardia, but at higher doses, epoprostenol causes reflex tachycardia in response to direct vasodilation and hypotension. No major effects on cardiac conduction have been observed. Additional pharmacologic effects of epoprostenol in animals include bronchodilation, inhibition of gastric acid secretion, and decreased gastric emptying. No available chemical assay is sufficiently sensitive and specific to assess the in vivo human pharmacokinetics of epoprostenol.
Metabolism
Epoprostenol is metabolized to 2 primary metabolites: 6-keto-PGF1α (formed by spontaneous degradation) and 6,15-diketo-13,14-dihydro-PGF1α (enzymatically formed), both of which have pharmacological activity orders of magnitude less than epoprostenol in animal test systems. Fourteen additional minor metabolites have been isolated from urine, indicating that epoprostenol is extensively metabolized in humans.
Properties of Epoprostenol
| Boiling point: | 530.2±50.0 °C(Predicted) |
| Density | 1.221 |
| pka | 4.70±0.10(Predicted) |
Safety information for Epoprostenol
Computed Descriptors for Epoprostenol
Epoprostenol manufacturer
Sai Life Sciences Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 35121-78-9 Epoprostenol 98%View Details
35121-78-9 Epoprostenol 98%View Details
35121-78-9 -
 35121-78-9 98%View Details
35121-78-9 98%View Details
35121-78-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1