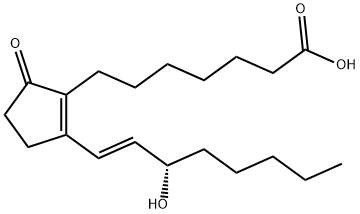
Prostaglandin E2
Synonym(s):Dinoprostone;Prostaglandin E2;PGE?;PGE2;Prostaglandin E2
- CAS NO.:363-24-6
- Empirical Formula: C20H32O5
- Molecular Weight: 352.47
- MDL number: MFCD00077861
- EINECS: 206-656-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
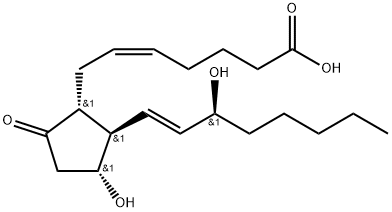
What is Prostaglandin E2 ?
Absorption
Absorbed at a rate of 0.3 mg per hour over 12 hours while the vaginal system is in place.
Toxicity
Oral, mouse: LD50 = 750 mg/kg; Oral, rat: LD50 = 500 mg/kg.
Description
Prostaglandin E2 (363-24-6; PGE2) is an endogenous prostaglandin derived from the action of cyclooxygenase on arachidonic acid. PGE2 has diverse biological actions in the areas of inflammation, cancer, immune modulation, fertility, smooth muscle relaxation and hematopoietic stem cell homeostasis. Prostaglandin E2 acts through four distinct receptors: EP1, EP2, EP3, EP4.
Description
Prostaglandins are hormone-like compounds that are produced in most animal tissues. They were discovered independently in seminal fluid in 1935 by Swedish physiologist and Nobel Prize winner Ulf von Euler and British physiologist M. W. Goldblatt.
In 1962, Swedish biochemist Sune Bergstr?m, another Nobel laureate, and his co-workers identified prostaglandin E2?(PGE2) in the seminal vesicle tissue of sheep. According to the?Merck Index, PGE2 “is the most common and most biologically active of mammalian prostaglandins.” In subsequent years, PGE2 was synthesized in the lab, first as a racemic mixture (1969–1970) and soon after as the naturally occurring (–)-isomer.
PGE2 has several medical uses, primarily during childbirth and in newborns. The US Food and Drug Administration approved its use in 1977; the uses include inducing labor, preventing postpartum bleeding, and keeping the ductus arteriosus closed in babies with congenital heart defects.
The body releases prostaglandins, including PGE2, as an inflammatory response to muscle injury. This sounds bad,?but the inflammation activates muscle stem cells that repair the damage, according to biologist Helen Blau and her research team at Stanford University. They found that treating injured mice with PGE2 accelerated repair of the damage.
Conversely, using a nonsteroidal anti-inflammatory drug (NSAID) such as ibuprofen or aspirin inhibited muscle repair in mice. This cautionary tale says that sometimes it’s best to leave the NSAID on the shelf and let inflammation do its work.
Chemical properties
White to pale yellowish-cream powder. Melting point 64-66°C, specific rotation -61°(26°C, c=1, THF). Easily hydrolyzed at pH<4 or pH>8. Soluble in chloroform, ethyl acetate, methanol, absolute ethanol and other organic solvents, slightly soluble in water.
Originator
Prostin E2,Upjohn,UK,1972
The Uses of Prostaglandin E2
The most common and most biologically potent of mammalian prostaglandins. Isolated from sheep prostate. Oxytocic; abortifacient.
The Uses of Prostaglandin E2
For use in cell culture applications for the study of prostaglandin regulated cell signaling and gene regulation.
Indications
For the termination of pregnancy during the second trimester (from the 12th through the 20th gestational week as calculated from the first day of the last normal menstrual period), as well as for evacuation of the uterine contents in the management of missed abortion or intrauterine fetal death up to 28 weeks of gestational age as calculated from the first day of the last normal menstrual period. Also used in the management of nonmetastatic gestational trophoblastic disease (benign hydatidiform mole). Other indications include improving the cervical inducibility (cervical "ripening") in pregnant women at or near term with a medical or obstetrical need for labor induction, and the management of postpartum hemorrhage.
Background
Dinoprostone is a naturally occurring prostaglandin E2 (PGE2). It has important effects in labour. It also stimulates osteoblasts to release factors which stimualtes bone resorption by osteoclasts. As a prescription drug it is used as a vaginal suppository, to prepare the cervix for labour and to induce labour.
What are the applications of Application
PGE2 is an extremely important eicosanoid involved in neuronal, metabolic, and immune system function
What are the applications of Application
Prostaglandin E2-biotinamide is A biotinylated PGE2 derivative designed to detect PGE2 bound in complexes
Definition
ChEBI: Prostaglandin F2alpha in which the hydroxy group at position 9 has been oxidised to the corresponding ketone. Prostaglandin E2 is the most common and most biologically potent of mammalian prostagland ns.
Manufacturing Process
Hexamethyldisilazane (1 ml) and trimethylchlorosilane (0.2 ml) are added
with stirring to a solution of PGA2 (250 mg) in 4 ml of tetrahydrofuran at 0°C
under nitrogen. This mixture is maintained at 5°C for 15 hours. The mixture is
then evaporated under reduced pressure. Toluene is added and evaporated
twice. Then the residue is dissolved in 6 ml of methanol, and the solution is
cooled to -20°C. Hydrogen peroxide (0.45 ml; 30% aqueous) is added. Then,
1N sodium hydroxide solution (0.9 ml) is added dropwise with stirring at -
20°C. After 2 hours at -20°C, an additional 0.3 ml of the sodium hydroxide
solution is added with stirring at -20°C. After another hour in the range -10°C
to -20°C, an additional 0.1 ml of the sodium hydroxide solution is added.
Then, 1.5 ml of 1 N hydrochloric acid is added, and the mixture is evaporated
under reduced pressure. The residue is extracted with ethyl acetate, and the
extract is washed successively with 1 N hydrochloric acid and
ine, dried with
anhydrous sodium sulfate and evaporated. The residue is dissolved in 5 ml of
diethyl ether. To this solution is added 0.5 ml of methanol and 0.1 ml of water.
Amalgamated aluminum made from 0.5 g of aluminum metal is then added in
small portions during 3 hours at 25°C. Then, ethyl acetate and 3 N
hydrochloric acid are added, and the ethyl acetate layer is separated and
washed successively with 1 N hydrochloric acid and
ine, dried with
anhydrous sodium sulfate, and evaporated. The residue is chromatographed
on 50 g of acid-washed silica gel, eluting first with 400 ml of a gradient of 50-
100% ethyl acetate in Skellysolve B, and then with 100 ml of 5% methanol in
ethyl acetate, collecting 25 ml fractions. Fractions 9 and 10 are combined and
evaporated to give 18 mg of 11β-PGE2. Fractions 17-25 are combined and
evaporated to give 39 mg of PGE2.
brand name
Cervidil (Controlled Therapeutics); Prepidil (Pharmacia & Upjohn); Prostin E2 (Pharmacia & Upjohn);Minprostin;Prostaglandin;Prostarmon e;Prostin vr pediatric.
Therapeutic Function
Oxytocic, Abortifacient
World Health Organization (WHO)
Dinoprostone, prostaglandin E2, was introduced into medicine in 1971 and is primarily used for cervical ripening during the induction of labour. It is available in various formulations for oral, parenteral and vaginal administration. Tablets, ampoules and vaginal dosage forms (tablets, pessaries, gel) remain registered in many countries.
General Description
PGE2 Dinoprostone (Prostin E2;Cervidil) is a naturally occurring prostaglandin that is administeredin a single dose of 10 mg by controlled-release(0.3 mg/hr) vaginal insert to induce cervical ripening. Use ofthis agent will potentiate the effects of oxytocin.
Biological Activity
Endogenous prostaglandin and primary product of arachidonic acid/cyclooxygenase pathway. Binds with high affinity to EP 1 , EP 2 , EP 3 and EP 4 receptors (K d values range between ~ 1-10 nM). Influences a wide range of processes including inflammation, smooth muscle relaxation, fertility and gastric mucosal integrity. Regulates vertebrate hematopoietic stem cell (HSC) homeostasis.
Biochem/physiol Actions
Most biologically active prostaglandin. PGE2 induces cervical ripening and parturition; mediates bradykinin-induced vasodilation; regulates adenylyl cyclase. Tumor cells that over-express cyclooxygenase 2 display increased invasiveness, angiogenesis, and resistance to apoptosis that may be due to the PGE2-induced expression of angiogenic factors and stabilization of the anti-apoptotic protein, survivin.The effect of PGE2 on the immune system is mixed. It inhibits T cell activation in vitro, suggesting it is an immunosuppressant. However, in vivo, it appears to effect expansion of the Th17 subset and differentiation of the Th1 subset of T helper cells, marking it as an immunoactivator.
Pharmacokinetics
Dinoprostone is equivalent to prostaglandin E2 (PGE2). It stimulates labor and delivery by stimulating the uterine, and thus terminates pregnancy. Dinoprostone is also capable of stimulating the smooth muscle of the gastrointestinal tract of man. This activity may be responsible for the vomiting and/or diarrhea that is not uncommon when dinoprostone is used to terminate pregnancy.
Safety Profile
Poison by subcutaneous and intravenous routes. Moderately toxic by ingestion and intraperitoneal routes. An experimental teratogen. Human reproductive effects by intravenous, intraplacental, and intravaginal routes: changes in the uterus, cervix and vagina; termination of pregnancy; and changes in ferthty. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
in vitro
pge2 can stimulate the gastric nonparietal secretion [3] and has been shown to regulate the function of many cell types including dendritic cells, macrophages, t and b lymphocytes leading to both pro- and anti-inflammatory effects [2].
in vivo
pge2 regulates many physiological systems including the gastrointestinal and immune systems. in the gastrointestinal tract, pge2 plays a protective role in maintaining the integrity of the gastric mucosa. pge2 has also been shown to play a role in the maintenance of blood pressure, particularly in the setting of salt overload [2].
Metabolism
Rapid metabolism of dinoprostone occurs primarily in the local tissues; any systemic absorption of the medication is cleared mainly in the maternal lungs and, secondarily, at sites such as the liver and kidneys.
Storage
-20°C
References
1) Healy (1990) Progesterone receptor antagonist and prostaglandins in human fertility regulation: a clinical review; Reprod. Fertil. Dev. 2 477
2) Greenhough et al. (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment; Carcinogenesis, 30 377
3) Kalinski (2012) Regulation of Immune Responses by Prostaglandin E2; J. Immunol., 188 21
4) North et al. (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis; Nature, 447 1007
5) Hoggatt et al. (2013) Differential stem- and progenitor-cell trafficking by prostaglandin E2; Nature, 495 365
6) Coleman et al. (1994) Classification of prostanoid receptors: Properties, distribution and structure of the receptors and their subtypes; Pharmacol. Rev. 46 205
Properties of Prostaglandin E2
| Melting point: | 66-68 °C |
| Boiling point: | 406.07°C (rough estimate) |
| alpha | -85.5 º (c=1, C2H5OH) |
| Density | 1.0601 (rough estimate) |
| refractive index | 1.6120 (estimate) |
| storage temp. | -20°C |
| solubility | ethanol: 1 mg/mL |
| form | powder |
| pka | pKa 4.77± 0.09(H2O,t=25±0.1,I=0.1(NaCl)) (Uncertain) |
| color | Clear yellow to amber |
| Water Solubility | insoluble |
| Merck | 14,7877 |
| BRN | 4709356 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 363-24-6(CAS DataBase Reference) |
Safety information for Prostaglandin E2
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Prostaglandin E2
| InChIKey | XEYBRNLFEZDVAW-ARSRFYASSA-N |
Prostaglandin E2 manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![[5,6(N)-3H]PROSTAGLANDIN E1](https://img.chemicalbook.in/CAS/GIF/20045-36-7.gif)
You may like
-
 363-24-6 Dinoprostone 98%View Details
363-24-6 Dinoprostone 98%View Details
363-24-6 -
 Dinoprostone 363-24-6 98%View Details
Dinoprostone 363-24-6 98%View Details
363-24-6 -
 Dinoprostone 363-24-6 98%View Details
Dinoprostone 363-24-6 98%View Details
363-24-6 -
 Dinoprostone 99%View Details
Dinoprostone 99%View Details -
 Prostaglandin E2 CAS 363-24-6View Details
Prostaglandin E2 CAS 363-24-6View Details
363-24-6 -
 Dinoprostone CAS 363-24-6View Details
Dinoprostone CAS 363-24-6View Details
363-24-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
