Gadoteric acid
- CAS NO.:72573-82-1
- Empirical Formula: C16H25GdN4O8
- Molecular Weight: 558.65
- MDL number: MFCD00867227
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 11:39:26
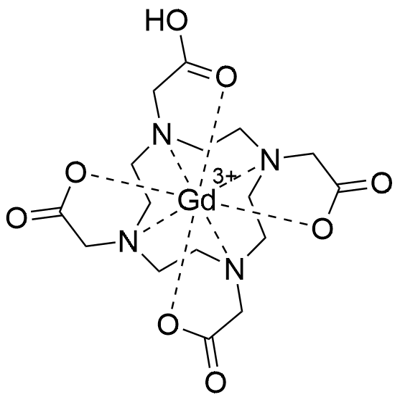
What is Gadoteric acid?
Toxicity
The most frequent adverse reactions in clinical studies were nausea, headache, injection site pain, injection site coldness, and burning sensation. Nephrogenic Systemic Fibrosis has occurred in patients with impaired elimination of GBCAs.
The Uses of Gadoteric acid
Gadoteric Acid is used for magnetic resonance imaging.
Background
Gadoteric acid is a macrocycle-structured gadolinium-based MRI contrast agent. It is composed of the organic acid DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) used for its chelating properties, and gadolinium (Gd3+). As a paramagnetic molecule, gadoterate develops a magnetic moment when placed in a magnetic field. This magnetic moment enhances the relaxation rates of water protons in its vicinity, leading to an increase in signal intensity (brightness) of tissues. More specifically, it reduces the T1 relaxation time (and to some extent the T2 and T2* relaxation times) in NMR, which is the source of its clinical utility. Increased signal brightness allows it to be used in imaging of blood vessels and of inflamed or diseased tissue where the blood vessels become 'leaky'.
Gadoteric acid, as the FDA approved product Dotarem, is indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (2 years of age and older) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.
Indications
Gadoteric acid is indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (2 years of age and older) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.
Definition
ChEBI: A gadolinium coordination entity consisting of DOTA in which the four amino groups are bound to a central gadolinium atom. It is used (as its meglumine salt) in magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues of patie ts ages 2 years and older, to detect and visualise areas with disruption of the blood brain barrier and/or abnormal vascularity of the central nervous system.
Pharmacokinetics
Gadoterate affects proton relaxation times and consequently the MR signal, and the contrast obtained is characterized by the relaxivity of the gadoterate molecule. The relaxivity values for gadoterate are similar across the spectrum of magnetic field strengths used in clinical MRI (0.2 - 1.5 T). Gadoterate does not cross the intact blood-brain barrier and, therefore, does not enhance normal brain or lesions that have a normal blood-brain barrier, e.g. cysts, mature post-operative scars. However, disruption of the blood-brain barrier or abnormal vascularity allows distribution of gadoterate in lesions such as neoplasms, abscesses, and infarcts.
Metabolism
Gadoterate is not known to be metabolized.
Properties of Gadoteric acid
| Melting point: | >300°C |
| storage temp. | Refrigerator |
| solubility | Water (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for Gadoteric acid
Computed Descriptors for Gadoteric acid
Gadoteric acid manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran







You may like
-
 72573-82-1 Gadoteric Acid 99%View Details
72573-82-1 Gadoteric Acid 99%View Details
72573-82-1 -
 72573-82-1 98%View Details
72573-82-1 98%View Details
72573-82-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1