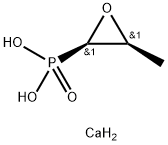
Fosfomycin
- CAS NO.:23155-02-4
- Empirical Formula: C3H7O4P
- Molecular Weight: 138.06
- MDL number: MFCD00242804
- EINECS: 245-463-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-25 16:21:11
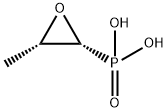
What is Fosfomycin?
Absorption
Fosfomycin is a low molecular weight and hydrophilic drug. When administered orally, fosfomycin is rapidly absorbed in the small intestine and distributed widely to the tissues. The oral bioavailability ranges from 34-58%. Co-administration of fosfomycin with food decreases gastrointestinal absorption to approximately 30%. The reported AUC = 145-228 mg x h/L, while the reported Cmax = 26.1 (?9.1) mcg/mL.
Toxicity
Acute toxicology studies have found that oral fosfomycin doses 50-125 times the human therapeutic dose were well-tolerated in rats and mice, resulted in minor and transient watery stools in rabbits, and caused diarrhea with anorexia in dogs 2-3 days after single-dose administration.
In humans, symptoms of overdose have included impaired hearing, vestibular loss, general decline in taste perception, and metallic taste. In the event of overdose, the patient should be managed with symptomatic and supportive measures.
Description
Fosfomycin is unique in possessing a simple epoxide ring and has a broad activity spectrum against gram-positive and gramnegative bacteria .
Chemical properties
Water-soluble crystals.
Originator
Fosfocin,Crinos,Italy,1977
The Uses of Fosfomycin
Antibacterial.
Background
Fosfomycin was discovered in 1969 by scientists at the Spanish Penicillin and Antibiotics Company and is produced by Streptomyces fradiae. It may also be produced synthetically and is commercially available as the disodium salt for intravenous administration and as the calcium or trometamol salt for oral administration. In terms of chemical structure, fosfomycin is a phosphoenolpyruvate analog and contains a phosphonic group and an epoxide ring.
Due to its ease of administration as a single 3-gram oral dose and desirable safety profile, fosfomycin has largely become a first-line therapeutic option for the treatment of uncomplicated urinary tract infections (UTIs) in females. Despite being FDA approved only for urinary tract infections, fosfomycin actually has a broad spectrum of activity and is active against both gram-positive and gram-negative bacteria. As such there is great interest in exploring the usefulness of fosfomycin for indications beyond the treatment of UTIs.
Indications
Fosfomycin is indicated for the treatment of uncomplicated cases of cystitis caused by susceptible strains of Escherichia coli and Enterococcus faecalis. Fosfomycin is not officially indicated for the treatment of pyelonephritis or perinephric abscess, although there have been reported cases of off-label usage in these situations.
Definition
ChEBI: A phosphonic acid having an (R,S)-1,2-epoxypropyl group attached to phosphorus.
Manufacturing Process
(A) The preparation of [(1-chloroethoxy)chloromethyl]phosphonic acid:
Acetaldehyde (1.1 mol) and hydroxymethylphosphonic acid (1 mol) in 500 ml
of benzene are saturated with hydrogen chloride gas at 10°C to 15°C. The
mixture is aged at 25°C for 24 hr, the solvent distilled out in vacuo and the
residue flushed three times with benzene to remove all traces of hydrogen chloride. The residue is taken up in benzene (500 ml), treated with tert-butyl
hypochlorite (0.8 mol) and azobisisobutyronitrile (0.8 mm) at 40°C until
titration shows the absence of hypochlorite and the solution is then
evaporated to yield [(1-chloroethoxy)chloromethyl] phosphonic acid in the
form of an oil.
(B) The preparation of (cis-1,2-epoxypropyl)phosphonic acid: [(1-
chloroethoxy)chloromethyl] phosphonic acid (1.0 g) is added with stirring to
tetrahydrofuran (50 ml) to which has been added a crystal of iodine and a
zinc-copper couple (15.0 g). The mixture is then heated under reflux for 24 hr
and the resulting solution filtered to yield (cis-1,2-epoxypropyl)-phosphonic
acid.
There is also a fermentation route to Fosfomycin as noted by Kleeman and
Engel.
Therapeutic Function
Antibiotic
Biological Activity
Fosfomycin shows antibacterial activity against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa andSerratiamarcescens,andβ-lactam-resistant Staphylococcus aureus. Its mechanism of action is probably the inhibition of cell-wall synthesis. It shows no cross-resistance with other classes of antibiotics.
Biological Activity
Fosfomycin shows antibacterial ac tivity against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa andSerratiamarcescens,andβ-lactam-resistant Staphylococcus aureus. Its mechanism of action is probably the inhibition of cell-wall synthesis. It shows no cross-resistance with other classes of antibiotics.
Pharmacokinetics
Although used primarily to treat urinary tract infections, fosfomycin has been shown to act synergistically with other antibiotics against clinically relevant bacteria. There is also growing interest in the potential of fosfomycin to treat more complex infections since it has retained activity against many difficult-to-treat strains of bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant enterobacteria. Further, since fosfomycin has a unique and singular mechanism of action, the risk of cross-resistance with other antibiotics is low.
Fosfomycin also demonstrates immunomodulating properties. For example, the antibiotic may influence components of the acute inflammatory cytokine response and enhances neutrophil phagocytic destruction of pathogens.
Fosfomycin penetrates biofilms effectively and is capable of not only reducing or eliminating microorganisms in biofilms but can also alter the biofilm structure.
Clinical Use
Phosphomycin, introduced in 1972, inhibits enolpyruvial transferase, an enzyme catalyzing an early step in bacterial cell wall biosynthesis. Inhibition results in reduced synthesis of peptidoglycan, an important component in the bacterial cell wall. Phosphomycin is bactericidal against Escherichi a coli and Enterobacter faecali s infections.
Drug interactions
Potentially hazardous interactions with other drugs
Metoclopramide: increases gastrointestinal motility
and therefore lowers the serum concentration and
urinary excretion of fosfomycin.
Metabolism
Fosfomycin is not metabolized and is predominantly excreted unchanged in the urine.
Metabolism
Fosfomycin undergoes no biotransformation and is excreted mainly unchanged through the kidneys. This results in very high urinary concentrations (up to 3 mg/mL) within 2-4 hours of a dose. Therapeutic concentrations of 200-300 mcg/mL in urine are usually maintained for at least 36 hours, and can last from 48-72 hours.
structure and hydrogen bonding
Fosfomycin's chemical structure is simple anduniqueamongantibiotics inhavinga C–P bond.
Properties of Fosfomycin
| Melting point: | 94°C |
| Boiling point: | 342.7±52.0 °C(Predicted) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| form | solid |
| pka | 3.20±0.40(Predicted) |
| CAS DataBase Reference | 23155-02-4(CAS DataBase Reference) |
Safety information for Fosfomycin
Computed Descriptors for Fosfomycin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 23155-02-4 Fosfomycin 99%View Details
23155-02-4 Fosfomycin 99%View Details
23155-02-4 -
 23155-02-4 98%View Details
23155-02-4 98%View Details
23155-02-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
