Disodium pamidronate
Synonym(s):3-Amino-1-hydroxypropylidene-bisphosphonic Acid, 2Na, Pentahydrate;Pamidronate disodium pentahydrate;Pamidronate, Disodium Salt - CAS 109552-15-0 - Calbiochem;Pamidronic acid disodium salt
- CAS NO.:109552-15-0
- Empirical Formula: C3H14NNaO8P2
- Molecular Weight: 277.08
- MDL number: MFCD01938386
- EINECS: 682-054-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
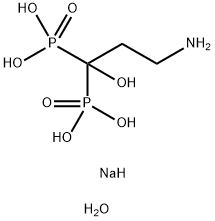
What is Disodium pamidronate?
Chemical properties
White or almost white, crystalline powder.
Originator
Aredia,Novartis,India
The Uses of Disodium pamidronate
Bone resorption inhibitor.
The Uses of Disodium pamidronate
Pamidronate Disodium Salt Pentahydrate is a calcium metabolism regulator.
What are the applications of Application
Pamidronate, Disodium Salt is a calcium metabolism regulator
Definition
ChEBI: Pamidronate disodium is a 1,1-bis(phosphonic acid).
Manufacturing Process
For a batch size of 5 L, 587.5 g (3.2 moles) of mannitol is dissolved in 3.5 L of water. Pamidronic acid (31.6 g, 0.133 moles) is mixed with a 1.0 L aliquot of the mannitol solution to form a slurry. The slurry is then transferred into the remainder of the mannitol solution, and stirred for at least 15 min. Aqueous 1 N sodium hydroxide (270 ml) is then added and the mixture is stirred until a clear, colorless solution results. The pH is then adjusted to 6.50.1 using either 1 M aqueous phosphoric acid or 1 N aqueous sodium hydroxide, as needed. The solution is then filtered through a 0.22 micron filter, and filled at 20°C into vials at 4.0 ml (4.172 g)/vial, under sterile conditions. The aqueous solution is frozen at -37°C and lyophilized (20 mbar, 20°-40°C) to yield 1,250 vials, each containing 30 mg of amorphous disodium pamidronate. The vials are sealed under positive nitrogen pressure. The disodium pamidronate is amorphous (noncrystalline) by X-ray diffraction and contains 0.7 wt-% water.
brand name
Aredia (Novartis).
Therapeutic Function
Bone resorption suppressant
Clinical Use
Pamidronate, a second-generation bisphosphonate, is 100-fold more potent than etidronate. It has been approved for the treatment of hypercalcemia of malignancy, for Paget's disease, and for osteolytic bone metastases of breast cancer and osteolytic lesions of multiple myeloma. When used to treat bone metastases, pamidronate decreases osteoclast recruitment, decreases osteoclast activity and increases osteoclast apoptosis. Erosive esophagitis has been reported with the use of pamidronate sodium.
Metabolism
Pamidronate is not metabolised, and about 20-55% of the dose is excreted in the urine unchanged within 72 hours; the remainder is mainly sequestered to bone and only very slowly eliminated.
Properties of Disodium pamidronate
| storage temp. | 2-8°C |
| solubility | Soluble in water, practically insoluble in methylene chloride. It is sparingly soluble in dilute mineral acids and dissolves in dilute alkaline solutions. |
| form | White solid |
| color | White to off-white |
| CAS DataBase Reference | 109552-15-0(CAS DataBase Reference) |
Safety information for Disodium pamidronate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Disodium pamidronate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

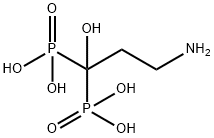




You may like
-
 40391-99-9 / 109552-15-0 Pamidronate Disodium 98%View Details
40391-99-9 / 109552-15-0 Pamidronate Disodium 98%View Details
40391-99-9 / 109552-15-0 -
 Pamidronate disodium CAS 109552-15-0View Details
Pamidronate disodium CAS 109552-15-0View Details
109552-15-0 -
 Pamidronate, Disodium Salt CAS 109552-15-0View Details
Pamidronate, Disodium Salt CAS 109552-15-0View Details
109552-15-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
