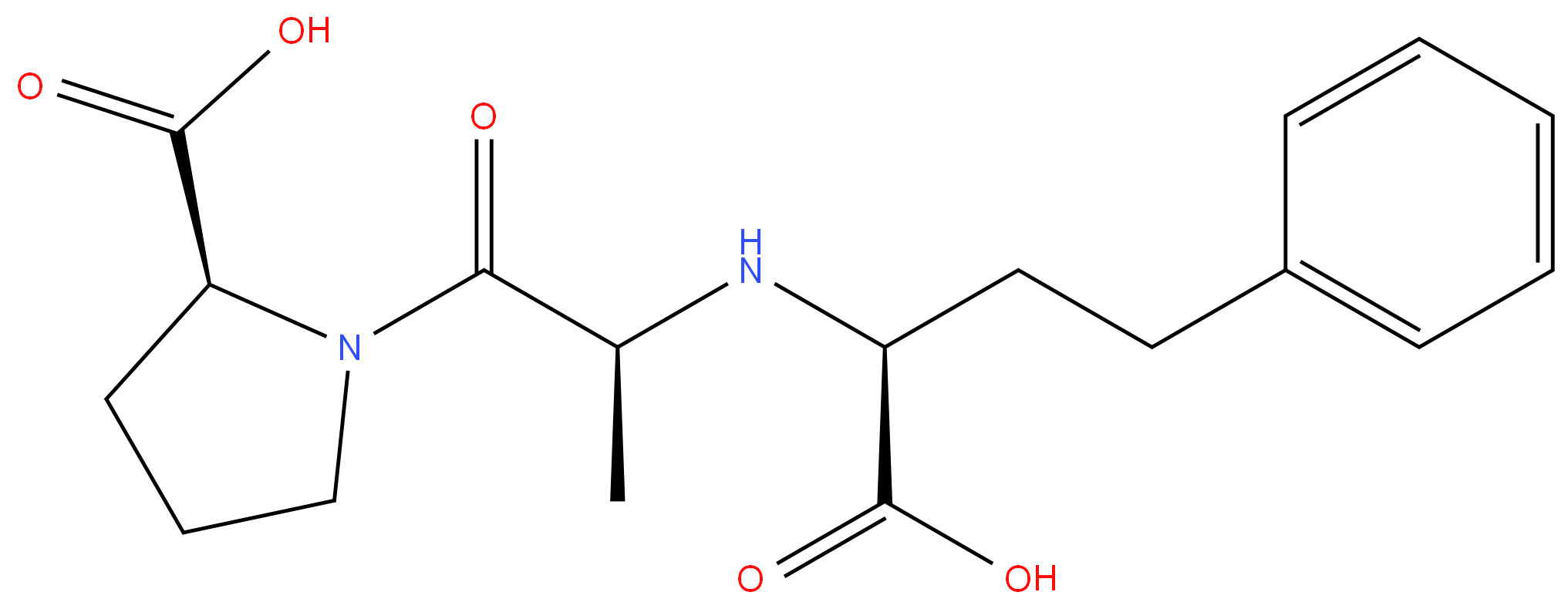
Enalaprilat
Synonym(s):(2S)-1-[(2S)-2-[[(1S)-1-Carboxy-3-phenyl-propyl]amino]propanoyl]pyrrolidine-2-carboxylic acid;(2S)-1-[(2S)-2-[[(1S)-1-Carboxy-3-phenyl-propyl]amino]propanoyl]pyrrolidine-2-carboxylic acid;N-[(1S)-1-Carboxy-3-phenylpropyl]-L -alanyl-L -proline;Enalaprilat dihydrate;Vasotec
- CAS NO.:76420-72-9
- Empirical Formula: C18H24N2O5
- Molecular Weight: 348.39
- MDL number: MFCD00865786
- EINECS: 278-459-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20

What is Enalaprilat?
Absorption
Enalaprilat is poorly absorbed following oral administration, and is therefore only available as an intravenous injection.
Toxicity
Adverse experiences occurring in 0.5 to 1.0 percent of patients in controlled clinical trials included: myocardial infarction, fatigue, dizziness, fever, rash and constipation.
Angioedema has also been reported in patients receiving enalaprilat, with an incidence higher in black than in non-black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with enalaprilat should be discontinued and appropriate therapy instituted immediately .
Rarer adverse effects that are less likely, but should be monitored for, include development of anaphylaxis, hypotension, agranulocytosis, hepatic failure, hyperkalemia, and persistent cough.
Furthermore, ACE inhibitors should be avoided during pregnancy as they can cause fetal and neonatal morbidity and death. When pregnancy is detected, ACE inhibitors should be discontinued as soon as possible. Use during the second and third trimesters of pregnancy has been associated with fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death.
Description
Enalaprilat is the diacid active metabolite of enalapril. As an injectable ACE inhibitor, it is reportedly useful in the treatment of uncomplicated accelerated hypertension and hypertensive emergencies. Enalaprilat is more effective in reducing blood pressure in the elderly than in the young, despite the fact that renin activity is lower in old age.
Chemical properties
White Crystalline Solid
Originator
Merck (USA)
The Uses of Enalaprilat
Enalapril is a ACE (angiotensin-converting enzyme) inhibitor, suggested to be more potent than Captopril (sc-200566). Studies indicate that Enalapril downregulates the expression of IL-β, IL-6, and NF-κB. Further studies show that Enalapril upregulates the expression of VEGF and heme oxygenase-1. Enalaprilat reports demonstrate that the compound has some antioxidant activity, which does not include the PKC-NADPH oxidase pathway.
The Uses of Enalaprilat
Active metabolite of Enalapril. A nonsulfhydryl dipeptide angiotensin converting enzyme (ACE) inhibitor
The Uses of Enalaprilat
primary hypertension
Background
Enalaprilat is the active metabolite of the orally available pro-drug, enalapril. Used in the treatment of hypertension, enalapril is an ACE inhibitor that prevents Angiotensin Converting Enzyme (ACE) from transforming angiotensin I into angiotensin II. As angiotensin II is responsible for vasoconstriction and sodium reabsorption in the proximal tubule of the kidney, down-regulation of this protein results in reduced blood pressure and blood fluid volume. Enalaprilat was originally created to overcome the limitations of the first ACE inhibitor, captopril, which had numerous side effects and left a metallic taste in the mouth. Removal of the problematic thiol group from captopril resulted in enalaprilat, which was then modified further with an ester to create the orally available pro-drug enalapril.
Enalaprilat is poorly orally available and is therefore only available as an intravenous injection for the treatment of hypertension when oral therapy is not possible.
Indications
Enalaprilat injection is indicated for the treatment of hypertension when oral therapy is not practical.
What are the applications of Application
Enalaprilat is an active metabolite of Enalapril
Definition
ChEBI: Enalapril in which the ethyl ester group has been hydrolysed to the corresponding carboxylic acid. Enalaprilat is an angiotensin-converting enzyme (ACE) inhibitor and is used (often in the form of its prodrug, enalapril) in the treatment of hypertension an heart failure, for reduction of proteinuria and renal disease in patients with nephropathies, and for the prevention of stroke, myocardial infarction, and cardiac death in high-risk patients. Unlike enalapril, enalaprilat is not absorbed by mouth but is g ven by intravenous injection, usually as the dihydrate.
brand name
Vasotec (Biovail);Renitec.
Pharmacokinetics
Enalaprilat injection results in the reduction of both supine and standing systolic and diastolic blood pressure, usually with no orthostatic component. Symptomatic postural hypotension is therefore infrequent, although it might be anticipated in volume-depleted patients. The onset of action usually occurs within fifteen minutes of administration with the maximum effect occurring within one to four hours. The abrupt withdrawal of enalaprilat has not been associated with a rapid increase in blood pressure. The duration of hemodynamic effects appears to be dose-related. However, for the recommended dose, the duration of action in most patients is approximately six hours. Following administration of enalapril, there is an increase in renal blood flow; glomerular filtration rate is usually unchanged. The effects appear to be similar in patients with renovascular hypertension.
Metabolism
Both enalapril and enalaprilat undergo renal excretion without further metabolism.
Properties of Enalaprilat
| Melting point: | 148-151°C |
| Boiling point: | 601.0±55.0 °C(Predicted) |
| alpha | D -67.0° (0.1M HCl) |
| Density | 1.286±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble or slightly soluble in water, sparingly soluble in methanol, practically insoluble in acetonitrile. |
| form | neat |
| pka | pKa 2.30 (Uncertain);3.39 (Uncertain) |
| Water Solubility | Soluble in water with heating (~60°C) |
| CAS DataBase Reference | 76420-72-9(CAS DataBase Reference) |
| EPA Substance Registry System | L-Proline, N-[(1S)-1-carboxy-3-phenylpropyl]-L-alanyl- (76420-72-9) |
Safety information for Enalaprilat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Enalaprilat
Enalaprilat manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
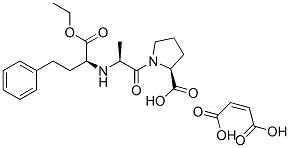
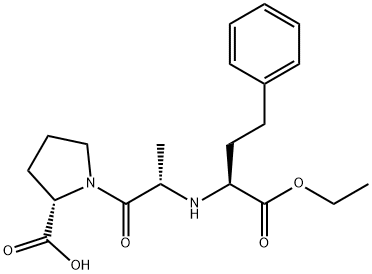
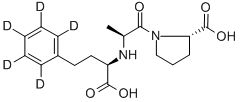

![(αS)-Cyclohexanebutanoic Acid α-[[(1S)-1-Carboxyethyl]aMino]
cyclohexanebutanoic Acid α-Ethyl Ester](https://img.chemicalbook.in/CAS/GIF/460720-14-3.gif)
You may like
-
 76420-72-9 Enalaprilat 98%View Details
76420-72-9 Enalaprilat 98%View Details
76420-72-9 -
 76420-72-9 98%View Details
76420-72-9 98%View Details
76420-72-9 -
 Enalaprilat CASView Details
Enalaprilat CASView Details -
 ENALAPRILAT 90 % AboveView Details
ENALAPRILAT 90 % AboveView Details
76420-72-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
