Idarubicin
Synonym(s):Idarubicin hydrochloride;Idamycin;IMI-30;4-Demethoxydaunorubicin hydrochloride;DMDR
- CAS NO.:58957-92-9
- Empirical Formula: C26H27NO9
- Molecular Weight: 497.49
- MDL number: MFCD00866457
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:30
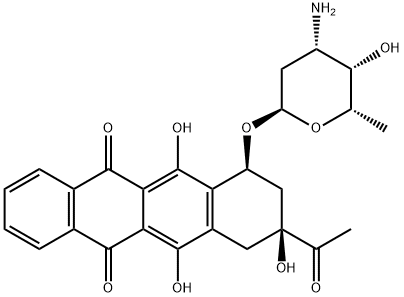
What is Idarubicin?
The Uses of Idarubicin
Antineoplastic.
Background
An orally administered anthracycline antineoplastic. The compound has shown activity against breast cancer, lymphomas and leukemias, together with the potential for reduced cardiac toxicity.
Indications
For the treatment of acute myeloid leukemia (AML) in adults. This includes French-American-British (FAB) classifications M1 through M7.
Indications
Idarubicin (Idamycin) differs from its parent compound, daunorubicin, by the absence of the methoxy group in the anthracycline ring structure. Its mechanisms of action and resistance are similar to those of doxorubicin and daunorubicin; however, it is more lipophilic and more potent than these other anthracyclines. Idarubicin undergoes extensive hepatic metabolism and biliary excretion. Adverse reactions of idarubicin are similar to those of its congeners.
Definition
ChEBI: Idarubicin is a monosaccharide derivative, an anthracycline antibiotic and a deoxy hexoside. It derives from a hydride of a tetracene.
Pharmacokinetics
Idarubicin is an antineoplastic in the anthracycline class. General properties of drugs in this class include: interaction with DNA in a variety of different ways including intercalation (squeezing between the base pairs), DNA strand breakage and inhibition with the enzyme topoisomerase II. Most of these compounds have been isolated from natural sources and antibiotics. However, they lack the specificity of the antimicrobial antibiotics and thus produce significant toxicity. The anthracyclines are among the most important antitumor drugs available. Doxorubicin is widely used for the treatment of several solid tumors while daunorubicin and idarubicin are used exclusively for the treatment of leukemia. Idarubicin may also inhibit polymerase activity, affect regulation of gene expression, and produce free radical damage to DNA. Idarubicin possesses an antitumor effect against a wide spectrum of tumors, either grafted or spontaneous. The anthracyclines are cell cycle-nonspecific.
Metabolism
Not Available
Properties of Idarubicin
| Boiling point: | 589.46°C (rough estimate) |
| Density | 1.3477 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| form | solid |
| pka | pKa 4.73± 0.21 (Uncertain) |
Safety information for Idarubicin
Computed Descriptors for Idarubicin
Idarubicin manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
