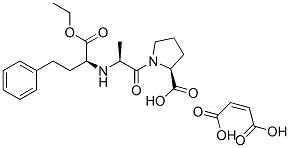
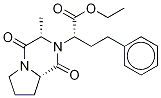
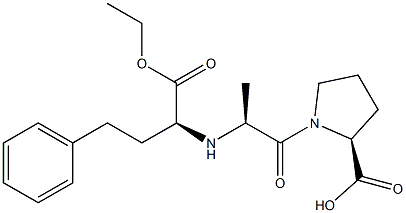
Enalapril maleate
Synonym(s):(S)-N-[1-(Ethoxycarbonyl)-3-phenylpropyl]-Ala-Pro maleate salt;Enalapril maleate salt
- CAS NO.:76095-16-4
- Empirical Formula: C24H32N2O9
- Molecular Weight: 492.52
- MDL number: MFCD00133304
- EINECS: 278-375-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
What is Enalapril maleate ?
Description
Enalapril maleate is the second angiotensin converting enzyme inhibitor to reach the marketplace. Like captopril, the first entry in this area, enalapril is useful in the treatment of hypertension and congestive heart failure. It has a longer effective half-life than captopril, allowing once or twice-daily dosing, and appears to have a somewhat lower incidence of side effects.
Chemical properties
White to Off-White Crystalline Powder
Originator
Merck (USA)
The Uses of Enalapril maleate
Enalapril maleate salt is used as a long-acting ACE inhibitor and antihypertensive, used to study diabetic angiopathy in diabetic rats and inhibition of ACE in hog plasma (I50=1.2nM). ACE inhibitors disturb the enin-angiotensin- aldosterone system. Additional ACE inhibitors include: Enalapril, Enalaprilat dihydrate, Enalaprilat, and Enalapril-d5 Maleate Salt, among others.
The Uses of Enalapril maleate
An antihypertensive. An angiotensin-converting enzyme (ACE) inhibitor.
The Uses of Enalapril maleate
sunscreen
What are the applications of Application
Enalapril Maleate is an angiotensin-converting enzyme inhibitor
Definition
ChEBI: The maleic acid salt of enalapril. It contains one molecule of maleic acid for each molecule of enalapril. Following oral administration, the ethyl ester group of enalapril is hydrolysed to afford the corresponding carboxylic acid, enalaprilat, an angioten in-converting enzyme (ACE) inhibitor. Enalapril is thus a prodrug for enalaprilat (which, unlike enalapril, is not absorbed by mouth), and its maleate is used in the treatment of hypertension and heart failure, for reduction of proteinuria and renal diseas in patients with nephropathies, and for the prevention of stroke, myocardial infarction, and cardiac death in high-risk patients.
Manufacturing Process
N-[1(S)-Ethoxycarbonyl-3-phenylpropyl]-L-alanyl-L-proline maleic acid salt
A mixture of 3 g of L-alanyl-L-proline, 5 g of ethyl 2-oxo-4-phenyl-butanoate,
13 g of 3A molecular sieves, and 3.6 g of Raney nickel in 85 ml of ethanol is
hydrogenated at 25°C and at 40 psig of hydrogen until uptake of hydrogen
ceases. The solids are filtered, washed with 80 ml of ethanol and the filtrates
are combined. Assay by high pressure liquid chromatography shows an 87:13
ratio of diastereoisomers in favor of the desired product. Ethanol is removed
under vacuum to afford an oil which is dissolved in 60 ml of water and 20 ml
of ethyl acetate. The pH of the stirred two-phase mixture is adjusted to 8.6
with 50% NaOH. The layers are separated and the water phase is extracted
with 2x20 ml of ethyl acetate. The water phase is adjusted to pH 4.25 with
hydrochloric acid, 12 g of NaCl is dissolved in the water, and product is
extracted with 5x12 ml of ethyl acetate. The extracts are combined and dried
with Na2SO4. The desired product, N-[1-(S)-ethoxycarbonyl-3-phenylpropyl]-
L-alanyl-L-proline, is crystallized as its maleate salt by addition of 1.86 g of
maleic acid. After stirring for 4 hours, the salt is filtered, washed with ethyl
acetate and dried to afford 5.2 g of pure product, melting point 150°-151°C.
brand name
RENITEN
Therapeutic Function
Antihypertensive
General Description
Enalapril maleate, 1-[N[(S)-1-carboxy-3-phenylpropyl]-L-alanyl]-L-proline 1 -ethyl estermaleate (Vasotec), is a long-acting ACE inhibitor. It requiresactivation by hydrolysis of its ethyl ester to form thediacid enalaprilat. Enalapril is devoid of the side effects ofrash and loss of taste seen with captopril. These side effectsare similar to those of the mercapto-containing drugpenicillamine. The absence of the thiol group in enalaprilmaleate may free it from these side effects. The half-life is11 hours.
Biochem/physiol Actions
A long-acting angiotensin-converting enzyme inhibitor.
Clinical Use
Angiotensin converting enzyme inhibitor:HypertensionHeart failure
Veterinary Drugs and Treatments
The principle use of enalapril/enalaprilat in veterinary medicine at
present is as a vasodilator in the treatment of heart failure. Recent
studies have demonstrated that enalapril, particularly when used
in conjunction with furosemide, does improve the quality of life
in dogs with heart failure. It is not clear, however, whether it has
any significant effect on survival times. It may also be of benefit
in treating the effects associated with valvular heart disease (mitral
regurgitation) and left to right shunts. It is being explored as
adjunctive treatment in chronic renal failure and protein losing
nephropathies.
While ACE inhibitors are a mainstay for treating hypertension in
humans, they have not been particularly useful in treating hypertension
in dogs or cats.
Drug interactions
Potentially hazardous interactions with other drugs Anaesthetics: enhanced hypotensive effect.Analgesics: antagonism of hypotensive effect and increased risk of renal impairment with NSAIDs; hyperkalaemia with ketorolac and other NSAIDsAntihypertensives: increased risk of hyperkalaemia, hypotension and renal failure with ARBs and aliskiren.Bee venom extract: possible severe anaphylactoid reactions when used togetherCiclosporin: increased risk of hyperkalaemia and nephrotoxicity.Cytotoxics: increased risk of angioedema with everolimus.Diuretics: enhanced hypotensive effect;hyperkalaemia with potassium-sparing diureticsESAs: increased risk of hyperkalaemia; antagonism of hypotensive effect.Gold: flushing and hypotension with sodium aurothiomalate.Lithium: reduced excretion, possibility of enhanced lithium toxicity.Potassium salts: increased risk of hyperkalaemiaTacrolimus: increased risk of hyperkalaemia and nephrotoxicity.
Metabolism
Following absorption, oral enalapril is rapidly and extensively hydrolysed to enalaprilat, a potent angiotensin converting enzyme inhibitor. Peak serum concentrations of enalaprilat occur about 4 hours after an oral dose of enalapril tablet, and the effective half-life is 11 hours. Excretion of enalaprilat is primarily renal. The principal components in urine are enalaprilat, accounting for about 40% of the dose, and intact enalapril.
Properties of Enalapril maleate
| Melting point: | 143-144,5°C |
| Boiling point: | 0°C |
| alpha | D25 -42.2° (c = 1 in methanol) |
| Flash point: | 0°C |
| storage temp. | 2-8°C |
| solubility | methanol: ≥50 mg/mL, clear, colorless to yellow |
| form | powder |
| pka | pKa1 3.0; pKa2 (25°) 5.4 |
| color | white to off-white |
| Water Solubility | Soluble in water, methanol, and ethanol. |
| λmax | 208nm(MeOH)(lit.) |
| Merck | 14,3567 |
| CAS DataBase Reference | 76095-16-4(CAS DataBase Reference) |
Safety information for Enalapril maleate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Enalapril maleate
| InChIKey | OYFJQPXVCSSHAI-QFPUQLAESA-N |
Enalapril maleate manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


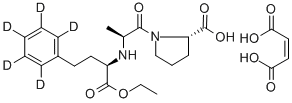
You may like
-
 76095-16-4 Enalapril Maleate 98%View Details
76095-16-4 Enalapril Maleate 98%View Details
76095-16-4 -
 Enalapril maleate 98%View Details
Enalapril maleate 98%View Details -
 Enalapril maleate 98%View Details
Enalapril maleate 98%View Details
76095-16-4 -
 Enalapril Maleate 98%View Details
Enalapril Maleate 98%View Details -
 Enalapril Maleate 98%View Details
Enalapril Maleate 98%View Details -
 Enalapril Maleate CAS 76095-16-4View Details
Enalapril Maleate CAS 76095-16-4View Details
76095-16-4 -
 Enalapril maleate salt 97% CAS 76095-16-4View Details
Enalapril maleate salt 97% CAS 76095-16-4View Details
76095-16-4 -
 Enalapril maleate CAS 76095-16-4View Details
Enalapril maleate CAS 76095-16-4View Details
76095-16-4
