Eplerenone
Synonym(s):Eplerenone;Inspra;Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, gamma-lactone, methyl ester, (7alpha,11alpha,17alpha)-;Eplerenone [USAN];Epoxymexrenone
- CAS NO.:107724-20-9
- Empirical Formula: C24H30O6
- Molecular Weight: 414.49
- MDL number: MFCD05662207
- EINECS: 600-850-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
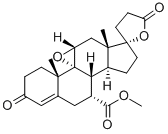
What is Eplerenone?
Absorption
The absolute bioavailability of eplerenone is unknown.
Toxicity
The most likely symptoms of human overdosage would be anticipated to be hypotension or hyperkalemia. However, no cases of human overdosage with eplerenone have been reported.
Description
Eplerenone is a mineralocorticoid receptor antagonist. It is selective for the mineralocorticoid receptor over glucocorticoid, androgen, progesterone, and estrogen receptors in radioligand binding assays (IC50s = 138, 6,920, 523, >10,000, and 5,702 nM, respectively). Eplerenone inhibits aldosterone-induced mineralocorticoid activity in a luciferase assay (IC50 = 122 nM). In vivo, eplerenone (100 mg/kg per day) reduces urinary albumin secretion and glomerulosclerosis in the Dahl salt-sensitive rat model of hypertension and nephropathy. It reduces myocardial IL-1β levels and collagen deposition, as well as improves left ventricular systolic dysfunction in a mouse model of acute myocardial infarction. Formulations containing eplerenone have been used in the treatment of hypertension and heart failure after myocardial infarction.
Description
Eplerenone derives its antihypertensive effect by blocking the binding of aldosterone at the mineralocorticoid receptor (MR). The drug, which was previously approved only for the oral treatment of hypertension, is now indicated to improve survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence congestive heart failure (CHF) after an acute myocardial infarction. Aldosterone is a key hormone in the renin-angiotensin-aldosterone system (RAAS), which is of critical importance in the development and progression of hypertension, cardiac remodeling and other cardiovascular diseases. The purpose of RAAS is to control sodium, potassium, and fluid volume balance. Aldosterone binds to MRs in both epithelial (e.g. kidney) and nonepithelial (e.g. heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms. The actions of aldosterone can be blocked by spironolactone (Aldactone ?), a relatively nonselective MR antagonist that has been used in clinical practice for many years. Eplerenone, a structural analog of spironolactone, is a highly selective MR antagonist, with significantly lower affinity for other nuclear receptors. It can be prepared by several related ways, with the key step being the introduction of 11-a-hydroxy group on the steroid scaffold via microbiological conversion. The presence of the 11-a-hydroxy group permits the derivation of the epoxy functionality found in eplerenone. Following oral administration, eplerenone is well absorbed and reaches peak plasma concentrations in~2 h. The bioavailability of eplerenone is 98% and it is cleared predominantly by CYP3A4 metabolism, with an elimination half-life of 4–6 h. Steady state is reached within two days. Eplerenone therapy is typically initiated with 25 mg once daily oral dosing and, if tolerated by the patient, titrated to 50 mg once daily. In a clinical study, eplerenone significantly reduced deaths in congestive heart failure patients after a heart attack, above and beyond standard therapy, including ACE inhibitors and β-blockers. The trial in more than 6600 hospitalized patients demonstrated a 15% reduction in the risk of death for eplerenone compared with placebo, in addition to standard treatment. The most commonly reported adverse events associated with eplerenone are hyperkalemia and increased creatine.
Chemical properties
White Solid
Originator
Ciba-Geigy (Novartis) (US)
The Uses of Eplerenone
Selective aldosterone receptor antagonist (SARA), structurally similar to Spiranolactone. Eplerenone is used alone or in combination with other medications to treat high blood pressure. Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It works by blocking the action of aldosterone, a natural substance in the body that raises blood pressure.
The Uses of Eplerenone
Eplerenone is an aldosterone antagonist with an IC50 of 0.36 μM. It is used as an adjunct in the management of chronic heart failure. It is similar to the diuretic spironolactone, though it may be more specific for the mineralocorticoid receptor and is sp
The Uses of Eplerenone
anticancer agent
Indications
For improvement of survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence of congestive heart failure after an acute myocardial infarction.
Background
Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors.
What are the applications of Application
Eplerenone is an MCR antagonist
Definition
ChEBI: Eplerenone is a steroid acid ester, a methyl ester, an oxaspiro compound, a gamma-lactone, an organic heteropentacyclic compound, a 3-oxo-Delta(4) steroid and an epoxy steroid. It has a role as an aldosterone antagonist and an antihypertensive agent. It derives from a hydride of a pregnane.
brand name
Inspra (Searle).
General Description
Eplerenone, 9,11α-epoxy-17α-hydroxy-3-oxopregn-4-ene-7α,21-dicarboxylic acid, γ-lactone,methyl ester (Inspra), is a newer aldosterone antagonist that isused for the treatment of hypertension.
Biological Activity
Selective mineralocorticoid (aldosterone) receptor antagonist (IC 50 = 360 nM). Displays > 27-fold selectivity over androgen, progesterone and estrogen receptors (IC 50 > 10 μ M). Orally active antihypertensive in vivo .
Biochem/physiol Actions
Eplerenone is an aldosterone antagonist more specific for the mineralocorticoid receptor than spironolactone (S3378), having lower affinity for progesterone, androgen, and glucocorticoid receptors.
Pharmacokinetics
Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors.
Clinical Use
A newer drug, eplerenone, has a structure similar to that of spironolactone and a similar mechanism of action. It was initially approved for use in the treatment of hypertension but it can now be used in the treatment of patients with left ventricular systolic dysfunction and congestive heart failure after myocardial infarction.
Drug interactions
Potentially hazardous interactions with other drugsACE inhibitors or AT-II antagonists: enhanced hypotensive effect; risk of severe hyperkalaemia.Anti-arrhythmics: concentration increased by amiodarone - reduce eplerenone dose.amiodarone - reduce eplerenone dose. Antibacterials: concentration increased by clarithromycin and telithromycin - avoid; concentration increased by erythromycin - reduce eplerenone dose; concentration reduced by rifampicin - avoid; avoid with lymecycline; increased risk of hyperkalaemia with trimethoprim.Antidepressants: concentration reduced by St John’s wort - avoid; increased risk of postural hypotension with tricyclics; enhanced hypotensive effect with MAOIs.Antiepileptics: concentration reduced by carbamazepine, fosphenytoin, phenytoin, phenobarbital and primidone - avoid.Antifungals: concentration increased by itraconazole and ketoconazole - avoid; concentration increased by fluconazole - reduce eplerenone dose.Antihypertensives: enhanced hypotensive effect, increased risk of first dose hypotensive effect with post-synaptic alpha-blockers.Antivirals: concentration increased by ritonavir - avoid; concentration increased by saquinavir - reduce eplerenone doseCiclosporin: increased risk of hyperkalaemia and nephrotoxicityCytotoxics: increased risk of nephrotoxicity and ototoxicity with platinum compounds.NSAIDs: increased risk of hyperkalaemia (especially with indometacin); increased risk of nephrotoxicity; antagonism of diuretic effect.Potassium salts: increased risk of hyperkalaemia.Lithium: reduced lithium excretion - avoidTacrolimus: increased risk of hyperkalaemia and nephrotoxicity.CYP3A4 inhibitors: Do not exceed a dose of 25 mg daily for eplerenone.CYP3A4 inducers: reduced eplerenone concentration - avoid.
Metabolism
Eplerenone is metabolized primarily by CYP3A4, however, no active metabolites have been identified in human plasma.
Metabolism
Eplerenone metabolism is primarily mediated via CYP3A4. No active metabolites of eplerenone have been identified in human plasma. Less than 5% of an eplerenone dose is recovered as unchanged drug in the urine and faeces. Following a single oral dose of radiolabelled drug, approximately 32% of the dose was excreted in the faeces and approximately 67% was excreted in the urine
Storage
Room temperature
Properties of Eplerenone
| Melting point: | 241-243°C |
| Boiling point: | 597.9±50.0 °C(Predicted) |
| alpha | D +5° (c = 0.437 in chloroform) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | Store at RT |
| solubility | DMSO: soluble2mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| λmax | 240nm(lit.) |
| Merck | 14,3625 |
Safety information for Eplerenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Eplerenone
| InChIKey | UZZAHAKTLYRDOK-MGIDJNSKSA-N |
| SMILES | [C@@]123[C@@]4(C)CCC(=O)C=C4C[C@@H](C(OC)=O)[C@@]1([H])[C@]1([H])CC[C@]4(CCC(=O)O4)[C@]1(C[C@@]2([H])O3)C |&1:0,1,10,15,17,21,27,29,r| |
Eplerenone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Eplerenone 98%View Details
Eplerenone 98%View Details
107724-20-9 -
 Eplerenone 107724-20-9 98%View Details
Eplerenone 107724-20-9 98%View Details
107724-20-9 -
 107724-20-9 98%View Details
107724-20-9 98%View Details
107724-20-9 -
 Eplerenone 98%View Details
Eplerenone 98%View Details
107724-20-9 -
 Eplerenone 99%View Details
Eplerenone 99%View Details -
 Eplerenone 97% CAS 107724-20-9View Details
Eplerenone 97% CAS 107724-20-9View Details
107724-20-9 -
 Eplerenone CAS 107724-20-9View Details
Eplerenone CAS 107724-20-9View Details
107724-20-9 -
 Eplerenone CAS 107724-20-9View Details
Eplerenone CAS 107724-20-9View Details
107724-20-9
