Enalapril
Synonym(s):(S)-(−)-1-[N-(1-Ethoxycarbonyl-3-phenylpropyl)-L -alanyl]-L proline
- CAS NO.:75847-73-3
- Empirical Formula: C20H28N2O5
- Molecular Weight: 376.45
- MDL number: MFCD00865774
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
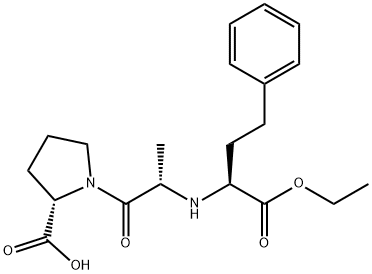
What is Enalapril?
Absorption
Following oral administration, the peak plasma concentrations (Cmax) of enalapril is achieved within 1 hour post dosing while the Cmax of enalaprilat occurs at three to four hours post dosing. The steady-state is achieved by the fourth daily dose and there is no accumulation with repeated dosing. However, accumulation of enalaprilat may occur in patients with creatinine clearance less than 30 mL/min. Food intake is reported to have a minimal effect on drug absorption. Following oral administration, about 60% of enalapril was absorbed. Bioavailability of enalapril averaged about 40% when intravenous enalaprilat was used as a reference standard.
Toxicity
LD50 and Overdose
Oral LD50 in rats is 2973 mg/kg. Lethality was observed with single oral doses of enalapril above 1000 mg/kg in mice and greater than or equal to 1775 mg/kg in rats. Serum enalaprilat levels 100- and 200-fold higher than usually seen after therapeutic doses have been reported after ingestion of 300 mg and 440 mg of enalapril, respectively. While there is limited data about enalapril overdose in humans, overdosage may result in marked hypotension and stupor based on the pharmacological properties of the drug. Most common adverse effects of enalapril include cough, hypotension, stupor, headache, dizziness and fatigue. If hypotension is seen, usual treatment of intravenous infusion of normal saline solution is recommended. Enalaprilat may be removed from systemic circulation with the use of hemodialysis. It has been removed from neonatal circulation by peritoneal dialysis.
Nonclinical toxicology
Maternal and fetal toxicity occudred in some rabbits treated with enalapril at doses of 1 mg/kg/day or more. There was no fetotoxicity, expressed as a decrease in average fetal weight, or teratogenicity in rats treated with enalapril at doses up to 200 mg/kg/day, which is about 333 times the maximum human dose. In mice and rats receiving enalapril at doses ranging from 90 to 180 mg/kg/day, there was no evidence of a tumorigenic effect. Neither enalapril or its active metabolite were shown to be mutagenic or genotoxic in in vitro and in vivo studies. There were no adverse effects on reproductive performance of male and female rats treated with up to 90 mg/kg/day of enalapril.
Use in special populations
Caution is warranted in patients who are concurrently using another ACE inhibitors with enalapril, as there have been incidences of agranulocytosis with the use of captopril, which is another ACE inhibitor. This adverse event may be particularly significant in patients with renal impairment or collagen vascular disease. As enalapril and enalaprilat were shown to be secreted in human milk in trace amounts, the use of enalaprilat in nursing women is not recommended. Significant fetal transfer occurs with enalapril and enalaprilat thus the use of the drug in pregnant women should be strongly avoided. Caution is advised when enalapril is used in patients who are elderly or with renal impairment, as dosage adjustments may be appropriate. The antihypertensive effect of angiotensin converting enzyme inhibitors is generally lower in individuals of African descent, usually a low-renin hypertensive population.
The Uses of Enalapril
Enalapril is used in the treatment of hypertension, diabetic nephropathy, and some types of chronic heart failure. It has been proven to protect the function of the kidneys in hypertension, heart failure, and diabetes, and may be used in the absence of hypertension for its kidney protective effects. It is widely used in chronic kidney failure.
The Uses of Enalapril
angiotensin converting enzyme inhibitor
The Uses of Enalapril
It is used for hypertension and chronic cardiac insufficiency.
Indications
Indicated for the management of essential or renovascular hypertension as monotherapy or in combination with other antihypertensive agents, such as thiazide diuretics, for an additive effect.
Indicated for the treatment of symptomatic congestive heart failure, usually in combination with diuretics and digitalis.
Indicated for the management of asymptomatic left ventricular dysfunction in patients with an ejection fraction of ≤ to 35 percent to decrease the rate of development of overt heart failure and the incidence of hospitalization for heart failure.
Background
Enalapril is a prodrug belonging to the angiotensin-converting enzyme (ACE) inhibitor drug class that works on the renin-angiotensin-aldosterone system, which is responsible for the regulation of blood pressure and fluid and electrolyte homeostasis. Enalapril is an orally-active and long-acting nonsulphydryl antihypertensive agent that suppresses the renin-angiotensin-aldosterone system to lower blood pressure. It was developed from a targeted research programmed using molecular modelling. Being a prodrug, enalapril is rapidly biotransformed into its active metabolite, enalaprilat, which is responsible for the pharmacological actions of enalapril. The active metabolite of enalapril competitively inhibits the ACE to hinder the production of angiotensin II, a key component of the renin-angiotensin-aldosterone system that promotes vasoconstriction and renal reabsorption of sodium ions in the kidneys. Ultimately, enalaprilat works to reduce blood pressure and blood fluid volume.
Commonly marketed under the trade name Vasotec, enalapril was first approved by the FDA in 1985 for the management of hypertension, heart failure, and asymptomatic left ventricular dysfunction. It is also found in a combination product containing hydrochlorothiazide that is used for the management of hypertension. The active metabolite enalaprilat is also available in oral tablets and intravenous formulations for injection.
What are the applications of Application
Enalapril is an angiotensin-converting enzyme inhibitor
Definition
ChEBI: A dicarboxylic acid monoester that is ethyl 4-phenylbutanoate in which a hydrogen alpha to the carboxy group is substituted by the amino group of L-alanyl-L-proline (S-configuration).
Mechanism of action
Like captopril, enalapril selectively suppresses the rennin–angiotensin–aldosterone system, inhibits angiotensin-converting enzyme, and prevents conversion of angiotensin I into angiotensin II.
Pharmacokinetics
Enalapril is an antihypertensive agent that exhibits natriuretic and uricosuric properties. Enalapril lowers blood pressure in all grades of essential and renovascular hypertension, and peripheral vascular resistance without causing an increase in heart rate. Individuals with low-renin hypertensive population were still responsive to enalapril. The duration of hypertensive effect in the systolic and diastolic blood pressure persists for at least 24 hours following initial administration of a single oral dose, and repeated daily administration of enalapril confers an additional reduction in blood pressure and a steady-state antihypertensive response may take several weeks. In patients with severe congestive heart failure and inadequate clinical response to conventional antihypertensive therapies, treatment with enalapril resulted in improvements in cardiac performance as observed by a reduction in both preload and afterload, and improved clinical status long-term. Furthermore, enalapril was shown to increase cardiac output and stroke volume while decreasing pulmonary capillary wedge pressure in patients with congestive heart failure refractory to conventional treatment with digitalis and diuretics. In clinical studies, enalapril reduced left ventricular mass, and did not affect cardiac function or myocardial perfusion during exercise. Enalapril is not highly associated with the risk of bradycardia unlike most diuretics and beta-blockers and it does not produce rebound hypertension upon discontinuation of therapy.
Enalapril is not reported to produce hypokalaemia, hyperglycaemia, hyperuricaemia or hypercholesterolaemia. In the kidneys, enalapril was shown to increase renal blood flow and decrease renal vascular resistance. It also augmented the glomerular filtration rate in patients with a glomerular filtration rate less than 80 mL/min. When used in combination, enalapril was shown to attenuate the extent of drug-induced hypokalemia caused by hydrochlorothiazide and the antihypertensive effects of both drugs were potentiated.
Synthesis
Enalapril, (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline (22.7.12), is synthesized by reacting the benzyl ester of L-alanyl-L-proline with the ethyl ester of 3-benzoylacrylic acid, which forms the product 22.7.11, the reduction of which with hydrogen using a Raney nickel catalyst removes the protective benzyl group, giving the desired enalapril (22.7.12) [24]. Alternative methods of syntheses have also been proposed.
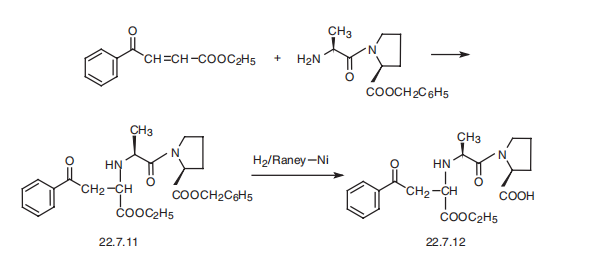
Metabolism
About 60% of the absorbed dose is extensively hydrolyzed to enalaprilat via de-esterification mediated by hepatic esterases. In humans, metabolism beyond bioactivation to enalaprilat is not observed.
Properties of Enalapril
| Melting point: | 142-147℃ |
| Boiling point: | 582.4±50.0 °C(Predicted) |
| Density | 1.204±0.06 g/cm3(Predicted) |
| RTECS | TW3665500 |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | pKa 2.97 (H2O t=25.0)(Approximate);5.35(H2O t=25.0)(Approximate) |
| Water Solubility | Soluble in water at 25mg/ml |
| CAS DataBase Reference | 75847-73-3(CAS DataBase Reference) |
| EPA Substance Registry System | L-Proline, N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl- (75847-73-3) |
Safety information for Enalapril
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Enalapril
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 75847-73-3 Enalapril 98%View Details
75847-73-3 Enalapril 98%View Details
75847-73-3 -
 75847-73-3 98%View Details
75847-73-3 98%View Details
75847-73-3 -
 Enalapril 98%View Details
Enalapril 98%View Details
75847-73-3 -
 Enalapril 75847-73-3 98%View Details
Enalapril 75847-73-3 98%View Details
75847-73-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
