Diantimony trioxide
Synonym(s):Diantimony trioxide;Antimony(III) oxide;JOS;MJD;SCA3
- CAS NO.:1309-64-4
- Empirical Formula: O3Sb2
- Molecular Weight: 291.52
- MDL number: MFCD00011214
- EINECS: 215-175-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-26 18:29:04
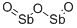
What is Diantimony trioxide?
Description
This hard shiny metal is often alloyed to other elements. It is used in various industrial fields, such as those making or using batteries, printing machines, bearing, textiles, and ceramics. It caused positive patch test reactions in two workers in a ceramics industry.
Chemical properties
White or gray mineral, sometimes pale red, white streak and adamantine or silky luster. Mohs hardness 2–3.
Chemical properties
Antimony trioxide is a noncombustible, odorless, white crystalline powder.
Physical properties
Occurs as colorless orthorhombic modifications, valentinite, or colorless cubic form, senarmontite; density 5.67 g/cm3 (valentinite), 5.20g/cm3 (senarmontite); cubic modification is dimeric consisting of Sb2O6 discrete molecules; refractive index 2.087; melts in the absence of oxygen at 656°C; boils at 1,550°C (sublimes); sublimes in vacuum at 400°C; very slightly soluble in water, insoluble in organic solvents; soluble in HCl, caustic alkalies and tartaric acid.
Occurrence
Antimony trioxide occurs in nature as minerals, valentinite [1317-98-2] and senarmontinite [12412-52-1]. It is used as a flame retardant in fabrics; as an opacifier in ceramics, glass and vitreous enamels; as a catalyst; as a white pigment in paints; as a mortar in the manufacture of tartar emetic; and in the production of metallic antimony.
The Uses of Diantimony trioxide
Antimony oxide, Sb2O3, is a nonreactive white pigment prepared from
metallic antimony using a similar technique to that used for the preparation
of zinc oxide.
Antimony oxide is widely used in the preparation
of fire retardant paint in conjunction with chlorine containing resins. On exposure to fire, the chlorine gas liberated by decomposition of the resin
component of the paint film reacts with the antimony oxide to produce
a vapor of antimony chloride that blankets the flames. Antimony oxide is also used to modify the heavy chalking characteristics of anatase form of titanium oxide.
The Uses of Diantimony trioxide
manufacture of tartar emetic; as paint pigment; in enamels and glasses; as mordant; in flame-proofing canvas.
The Uses of Diantimony trioxide
Antimony Trioxide (commonly referred to as antimony oxide), Sb2O3 is used to impart flame retardancy to plastics. Although antimony trioxide is found in nature, it is too impure to be used. Flameretardant grades of antimony oxides are manufactured from either antimony metal or the sulfide ore by oxidation in air at 600–800 °C. The particle size and chemical reactivity is determined by the processing conditions, enabling the production of several different grades. Antimony trioxide is from 99.0–99.9 wt % Sb2O3. The remainder consists of 0.4–0.01 wt % arsenic; 0.4–0.01, lead; 0.1–0.0001, iron; 0.005–0.0001, nickel; and 0.01–0.0001, sulfates. It is insoluble in water and the loss on drying at 110 °C is 0.1 wt % max.
Antimony trioxide has been used as a white pigment since ancient times. The pigmentation from antimony oxide in plastics can be controlled and adjusted by the judicious selection of a Sb2O3 grade having a specific particle size. The product with the smallest particle size and the narrowest particle-size range imparts the whitest color and highest opacity. Translucent plastics can be made by using low tint grades with relatively large particles.
Definition
A white insoluble solid. It is an amphoteric oxide with a strong tendency to act as a base. It can be prepared by direct oxidation by air, oxygen, or steam and is formed when antimony(III) chloride is hydrolyzed by excess boiling water.
Preparation
Antimony trioxide is obtained by roasting stibnite:
2 Sb2S3 + 9 O2 → 2Sb2O3 + 6SO2
Temperature and air feed is carefully controlled in the process to suppress any formation of antimony tetroxide (Sb2O4). Antimony trioxide is separated from any arsenic trioxide (As2O3) that may be present as an impurity by volatilization, as the latter is much more volatile than the former. It may be also prepared by alkaline hydrolysis of antimony trichloride and subsequent dehydration of hydrous oxide under controlled heating (rapid or vigorous heating may partially oxidize Sb(III) to Sb(V).
Antimony trioxide also may be made by heating the metallic element with oxygen or air. The volatilizing trioxide is condensed and collected.
General Description
Diantimony trioxide is a white crystalline solid. Diantimony trioxide is insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Diantimony trioxide is used to fireproof fabrics, paper and plastics, as a paint pigment and for many other uses.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
IDiantimony trioxide ignites and burns when heated in powdered form in air [Mellor 9:425 1946-47]. Reacts violentlhy with bromine trifluoride [Mellor Vol. 9 425.1939].
Hazard
Possible carcinogen during production.
Health Hazard
DUST: POISONOUS IF INHALED OR IF SKIN IS EXPOSED. If inhaled will cause coughing, difficult breathing or loss of consciousness. SOLID: POISONOUS IF SWALLOWED OR IF SKIN IS EXPOSED. If swallowed will cause dizziness, nausea, vomiting or loss of consciousness.
Fire Hazard
Not flammable.
Contact allergens
This hard shiny metal is often alloyed to other elements. It is used in various industrial fields such as batteries, printing machines, bearing, textile, and ceramics. It caused positive patch test reactions in two workers in the ceramics industry.
Biochem/physiol Actions
Machado-Joseph disease, also known as spinocerebellar ataxia-3, is an autosomal dominant neurologic disorder. The protein encoded by this gene contains (CAG)n repeats in the coding region, and the expansion of these repeats from the normal 13-36 to 68-79 is the cause of Machado-Joseph disease. There is a negative correlation between the age of onset and CAG repeat numbers. Alternatively spliced transcript variants encoding different isoforms have been described for this gene. [provided by RefSeq]
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intravenous and subcutaneous routes. Moderately toxic by other routes. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also ANTIMONY COMPOUNDS. When heated to decomposition it emits toxic Sb fumes. Incompatible with chlorinated rubber and heat of 21 6° and with BrF3.
Potential Exposure
It is used in flame-proofing, pigments and ceramics, to stain iron and copper; to decolorize glass; industrial chemical, dye, pigment, and printing ink.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from heat, strong oxidizers, acids. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHA Standard1910.1045.
Shipping
UN1549 Antimony compounds, inorganic, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Dissolve the trioxide in the minimum volume of dilute HCl, filter, and add six volumes of water to precipitate the basic antimonous chloride (free from Fe and Sb2O5). The precipitate is redissolved in dilute HCl, and added slowly, with stirring, to a boiling solution (containing a slight excess) of Na2CO3. The oxide is filtered off, washed with hot water, then boiled and filtered. The process is repeated until the filtrate gives no test for chloride ions. The product is dried in a vacuum desiccator [Schuhmann J Am Chem Soc 46 52 1924]. After one crystallisation (precipitation), the oxide from a Chinese source had: metal (ppm) Al (8), Ag (0.2), As (56), Cr (6), Ge (0.4), Mn (0.2), Na (16), Ni (2.2) Pb (2.4), Sn (0.4) and V (32). It sublimes in a vacuum at 400o, being yellow on heating and pale buff in colour on cooling. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 615-616 1963.] Aqua regia. This is prepared by adding slowly concentrated HNO3 (1 volume) to concentrated hydrochloric acid (3 volumes) in a glass container. This mixture is used to dissolve metals, including noble metals and alloys, as well as minerals and refractory substances. It is done by suspending the material and boiling (EFFICIENT FUME CUPBOARD — EYE PROTECTION] to dryness and repeating the process until the residue dissolves in H2O. If the aqua regia is to be stored for long periods it is advisable to dilute it with one volume of H2O which will prevent it from releasing chlorine and other chloro and nitrous compounds which are objectionable and toxic. Store it cool in a fume cupboard. However, it is good laboratory practice to prepare it freshly and dispose of it down the fume cupboard sink with copious amounts of water.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, halogenated acids, chlorinated rubber, bromine trifluoride. Reduction with hydrogen forms toxic antimony hydride.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Diantimony trioxide
| Melting point: | 655 °C (lit.) |
| Boiling point: | 1550 °C (lit.) |
| Density | 5.20 |
| vapor pressure | 13.3 hPa (660 °C) |
| Flash point: | 1550°C subl. |
| storage temp. | Store below +30°C. |
| solubility | 2.70mg/l |
| form | powder |
| color | White |
| Specific Gravity | 5.67 |
| Odor | wh. cubic or orthorhombic cryst., odorless |
| Water Solubility | Slightly soluble. <0.1 g/100 mL at 20 ºC |
| Merck | 14,711 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 1309-64-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Antimony trioxide(1309-64-4) |
| IARC | 2B (Vol. 47) 1989 |
| EPA Substance Registry System | Antimony trioxide (1309-64-4) |
Safety information for Diantimony trioxide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Diantimony trioxide
| InChIKey | MUBFITUCTVFSOJ-UHFFFAOYSA-L |
Diantimony trioxide manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 ANTIMONY TRIOXIDE 99.5% 99%View Details
ANTIMONY TRIOXIDE 99.5% 99%View Details -
 Antimony trioxide 99%View Details
Antimony trioxide 99%View Details -
 Antimony(III) oxide CAS 1309-64-4View Details
Antimony(III) oxide CAS 1309-64-4View Details
1309-64-4 -
 Antimony(III) oxide CAS 1309-64-4View Details
Antimony(III) oxide CAS 1309-64-4View Details
1309-64-4 -
 Antimony(III) oxide CAS 1309-64-4View Details
Antimony(III) oxide CAS 1309-64-4View Details
1309-64-4 -
 Antimony(III) oxide CAS 1309-64-4View Details
Antimony(III) oxide CAS 1309-64-4View Details
1309-64-4 -
 Antimony(III) oxide CAS 1309-64-4View Details
Antimony(III) oxide CAS 1309-64-4View Details
1309-64-4 -
 Antimony Trioxide CASView Details
Antimony Trioxide CASView Details
