Gallium(III) oxide
Synonym(s):Gallium trioxide
- CAS NO.:12024-21-4
- Empirical Formula: Ga2O3
- Molecular Weight: 187.44
- MDL number: MFCD00011020
- EINECS: 234-691-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
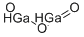
What is Gallium(III) oxide?
Description
Gallium(III) oxide (Ga2O3) is a wide band gap semiconductor that belongs to a family of transparent semiconducting oxides (TSO). It can form different polymorphs such as α-,β-, γ-, δ-, and ε-. Polycrystalline and nanocrystalline Ga2O3 can be prepared using several methods such as chemical vapor deposition, thermal vaporization, and sublimation, molecular beam epitaxy, melt growth, etc. It is widely used as a functional material in various applications including optoelectronics, chemical sensors, catalysis, semiconductor devices, field-effect transistors, and many others.
Chemical properties
white odourless powder
The Uses of Gallium(III) oxide
In semiconductors; gas sensing; catalysis. Nanostructures as blue and UV light emitters in optoelectronic device applications.
Gallium(III) oxide has been reported in applications in lasers, phosphors, and luminescent materials. Monoclinic ß-Ga2O3 is used in gas sensors and luminescent phosphors and can be applied to dielectric coatings for solar cells. This stable oxide has also shown potential for deep-ultraviolet transparent conductive oxides, and transistor applications. Thin Ga2O3 films are of commercial interest as gas sensitive materials. ß-Gallium(III) oxide is used in the production of Ga2O3-Al2O3 catalyst.
The Uses of Gallium(III) oxide
Gallium(III) oxide is used in vacuum deposition. It is useful for making semiconductor devices, gallium-alumina catalyst, gas sensors, luminescent phosphors and dielectric coatings of solar cells. It shows potential for developing deep-ultraviolet TCOs (Transparent Conductive Oxides) and transparent electrodes for ultraviolet optoelectronic devices. Recent studies report that gallium oxide can be a strong contender for power electronic devices for example, in ultrahigh-voltage power switching applications. Films made of gallium oxide have gained commercial interest owing to their gas sensitive characteristics, and glasses made with gallium oxide are the preferred optical materials for use in advanced technologies.
Production Methods
Gallium oxide is formed during the annealing or heating of semiconductors containing gallium in the microelectronics industry.
What are the applications of Application
Gallium(III) oxide is widely used as a host material for the fabrication of electroluminescent devices. For example, europium-doped Ga2O3 thin films can be used as a light-emitting layer to fabricate an optically transparent electroluminescent device.
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Starting material for the preparation of Sr2CuGaO3S, an example of a rare square pyramidal gallium.
Gallium(III) oxide is used in spectroscopic analysis and in preparing gallium arsenide for making semiconductors.
General Description
Odorless fine white powder. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
GALLIUM(III) OXIDE may react with acids. Heating (to 1292° F) with magnesium causes a violent reduction to metallic slate.
Health Hazard
SYMPTOMS: Symptoms of exposure to GALLIUM(III) OXIDE include reduction of red blood cells and platelets, anemia, skin rash and nausea.
Fire Hazard
Flash point data for GALLIUM(III) OXIDE are not available; however, GALLIUM(III) OXIDE is probably combustible.
References
Highly selective and rapid enrichment of phosphorylated peptides using gallium oxide‐coated magnetic microspheres for MALDI‐TOF‐MS and nano‐LC‐ESI‐MS/MS/MS analysis DOI:10.1002/PMIC.200700454
Development of gallium oxide power devices (Phys. Status Solidi A 1∕2014) DOI:10.1002/PSSA.201470201
Synthesis and characterization of morphologically different high purity gallium oxide nanopowders DOI:10.1007/S10853-007-1869-2
Structure, Morphology, and Optical Properties of Amorphous and Nanocrystalline Gallium Oxide Thin Films DOI:10.1021/JP311300E
Electrical and optical properties of deep ultraviolet transparent conductive Ga2O3/ITO films by magnetron sputtering DOI:10.1088/1674-4926/31/10/103001
Patty's Toxicology, 6 Volume Set, 6th Edition
Properties of Gallium(III) oxide
| Melting point: | 1740 °C |
| Density | 6.44 g/mL at 25 °C |
| refractive index | 1.92 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | soluble in hot acid solutions |
| form | Powder or Lump |
| color | White |
| Specific Gravity | 6.44 |
| Water Solubility | Soluble in acids. Insoluble in water |
| Merck | 14,4346 |
| Stability: | Stable. Incompatible with magnesium. |
| CAS DataBase Reference | 12024-21-4(CAS DataBase Reference) |
| EPA Substance Registry System | Gallium trioxide (12024-21-4) |
Safety information for Gallium(III) oxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Gallium(III) oxide
| InChIKey | QZQVBEXLDFYHSR-UHFFFAOYSA-N |
Related products of tetrahydrofuran
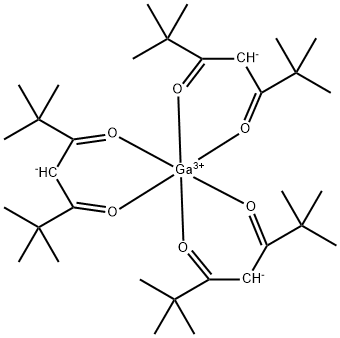
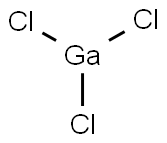
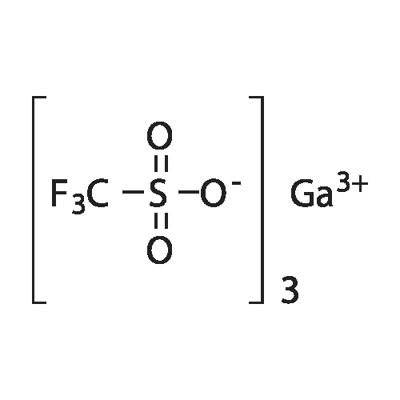
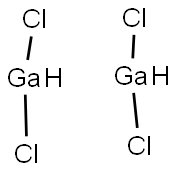
You may like
-
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4 -
 Gallium(III) oxide CAS 12024-21-4View Details
Gallium(III) oxide CAS 12024-21-4View Details
12024-21-4