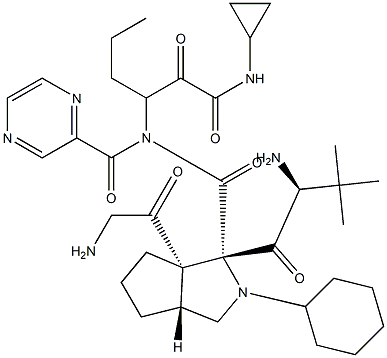
Daclatasvir
- CAS NO.:1009119-64-5
- Empirical Formula: C40H50N8O6
- Molecular Weight: 738.88
- MDL number: MFCD17129086
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
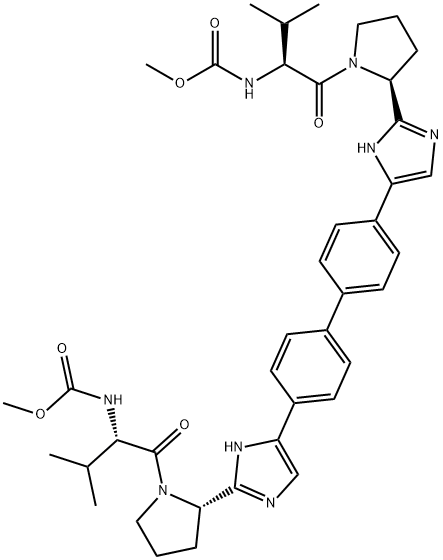
What is Daclatasvir?
Absorption
Studies demonstrated that peak plasma concentrations typically occurred within 2 hours after administration of multiple oral doses ranging from 1 - 100 mg once daily. Steady state is reached after approximately 4 days of once-daily daclatasvir administration. The absolute bioavailability of the tablet formulation is 67%.
Toxicity
The most common adverse effects experienced in patients undergoing daclatasvir and sofosbuvir therapy include headache, fatigue, nausea and diarrhea. Similar side effects are seen when ribavirin is added, in addition to rash, insomnia, anemia, dizziness and somnolence. There are postmarketing cases that link serious symptomatic bradycardia with Daklinza when used in conjunction with sofosbuvir and amiodarone. Coadministration of these three drugs is not recommended unless there are no other alternatives.
The Uses of Daclatasvir
Daclatasvir, inhibits the HCV protein NS5A, and thus can be used as a drug candidate for the treatment of hepatitis C (HCV).
Background
Daclatasvir is a direct-acting antiviral agent against Hepatitis C Virus (HCV) used for the treatment of chronic HCV genotype 1 and 3 infection. It is marketed under the name DAKLINZA and is contained in daily oral tablets as the hydrochloride salt form . Hepatitis C is an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients . Daclatasvir was the first drug with demonstrated safety and therapeutic efficacy in treating HCV genotype 3 without the need for co-administration of interferon or Ribavirin. It exerts its antiviral action by preventing RNA replication and virion assembly via binding to NS5A, a nonstructural phosphoprotein encoded by HCV. Binding to the N-terminus of the D1 domain of NS5A prevents its interaction with host cell proteins and membranes required for virion replication complex assembly. Daclatasvir is shown to target both the cis- and trans-acting functions of NS5A and disrupts the function of new HCV replication complexes by modulating the NS5A phosphorylation status . The most common critical NS5A amino acid substitutions that led to reduced susceptibility to daclatasvir therapy occured at position Q30 (Q30H/K/R) and M28 in genotype 1a patients and Y93H in genotype 3 patients.
According to 2017 American Association for the Study of Liver Diseases (AASLD), 60mg of daclatasvir is recommended with 400mg Sofosbuvir for genotype 1a/b patients with or without cirrhosis as second-line therapy. The same dosing regimen can be used as first-line therapy in patients with genotype 3 without cirrhosis and second-line therapy in genotype 3 patients with compensated cirrhosis. Combination therapies that include daclatasir can be used for challenging-to-treat patients who have HIV-1 coinfection, advanced cirrhosis, or post-liver transplant recurrence of HCV . The therapy is intended to cure or achieve a sustained virologic response (SVR12), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality .
Daclatasvir was FDA-approved in July 2015 for use with Sofosbuvir (Sovaldi) with or without Ribavirin to treat HCV genotype 1 and 3 infections. The SVR12 in HCV genotype 1a-infected treatment-na?ve subjects without and with cirrhosis undergoing daclatasvir and Sofosbuvir therapy were 88% and 99%, respectively . The same dosing regimen in treatment-na?ve patients with HCV genotype 3 infection with or without cirrhosis achieved SVR12 rates of 71% and 98%, respectively .
Indications
Indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic HCV genotype 1a/b or 3 infection. The dosing regimen of 60mg daclatasvir 60 mg with 400mg sofosbuvir once a day is recommended for both genontypes.
Resistance: Reduced susceptibility to daclatasvir was associated with the polymorphisms at NS5A amino acid positions M28, Q30, L31, and Y93 in genotypes 1a, 1b, and 3a patients. NS5A Resistance Testing is recommended for HCV genotype 1a-infected patients with cirrhosis prior to the initiaition of the treatment, as the risk of resistance development is higher in genotype 1a patients.
Definition
ChEBI: A member of the class of biphenyls that is a potent inhibitor of nonstructural protein 5A and is used (as its hydrochloride salt) for treatment of hepatitis C.
Pharmacokinetics
Daclatasvir is a direct-acting antiviral agent that targets the NS5A and causes a decrease in serum HCV RNA levels. It disrupts HCV replication by specifically inhibiting the critical functions of an NS5A protein in the replication complex . It is shown to cause downregulation of the hyperphosphorylation of NS5A. It does not appear to prolong the QT interval even when given at 3 times the maximum recommended dose.
Clinical Use
Inhibitor of nonstructural protein 5A (NS5A):
Treatment of chronic hepatitis C infection in
combination with other medication
Drug interactions
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Metabolism
Daclastavir is a substrate of CYP3A enzymes where its metabolism is predominantly mediated by CYP3A4 isoform. Oxidative pathways included δ-oxidation of the pyrrolidine moiety, resulting in ring opening to an aminoaldehyde intermediate followed by an intramolecular reaction between the aldehyde and the proximal imidazole nitrogen atom . High proportion of the drug in the plasma (greater than 97%) is in the unchanged form.
Metabolism
Dacarbazine (DTIC) is assumed to be inactive
Dacarbazine is extensively metabolised in the liver Dacarbazine is extensively metabolised in the liver
by the cytochrome P450 isoenzymes CYP1A2 and
CYP2E1 (and possibly in the tissues by CYP1A1)
to its active metabolite 5-(3-methyl-triazen-1-yl)-
imidazole-4-carboxamide (MTIC), which spontaneously
decomposes to the major metabolite 5-amino-imidazole-
4-carboxamide (AIC). About half of a dose is excreted in
the urine by tubular secretion; 50% as unchanged DTIC
and approximately 50% as AIC.
Properties of Daclatasvir
| Melting point: | >155oC (dec.) |
| Boiling point: | 1071.2±65.0 °C(Predicted) |
| Density | 1.249 |
| storage temp. | Refrigerator |
| solubility | Aqueous Acid (Slightly), Chloroform (Sparingly), DMSO (Sparingly), Methanol (Spa |
| form | Solid |
| pka | 10.92±0.46(Predicted) |
| color | White to Dark Yellow |
Safety information for Daclatasvir
Computed Descriptors for Daclatasvir
Daclatasvir manufacturer
Varanous Labs Pvt Ltd
BDR Pharmaceuticals International Pvt Ltd
Aspen Biopharma Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)

You may like
-
 1009119-64-5 Daclatasvir 98%View Details
1009119-64-5 Daclatasvir 98%View Details
1009119-64-5 -
 1009119-64-5 98%View Details
1009119-64-5 98%View Details
1009119-64-5 -
 Daclatasvir 1009119-64-5 98%View Details
Daclatasvir 1009119-64-5 98%View Details
1009119-64-5 -
 Daclatasvir 98% (HPLC) CAS 1009119-64-5View Details
Daclatasvir 98% (HPLC) CAS 1009119-64-5View Details
1009119-64-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1