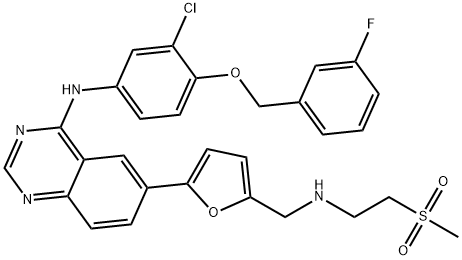
Lapatinib
Synonym(s):;Lapatinib;N-(3-Chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl)-4-quinazolinamine;N-(3-Chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-((2-methylsulfonylethylamino)methyl)-2-furyl)quinazolin-4-amine
- CAS NO.:231277-92-2
- Empirical Formula: C29H26ClFN4O4S
- Molecular Weight: 581.06
- MDL number: MFCD09264194
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Lapatinib?
Absorption
Absorption following oral administration of lapatinib is incomplete and variable.
Toxicity
There has been a report of one patient who took 3,000 mg of lapatinib for 10 days. This patient had grade 3 diarrhea and vomiting on day 10.
Description
Lapatinib, a new member of the 4-anilinoquinazoline class of RTK inhibitors
(RTKIs), was launched as an oral treatment for breast cancer.
Lapatinib has dual affinity for EGFR and HER2 tyrosine kinases. It is indicated
in combination with capecitabine for treating patients with advanced or
metastatic breast cancer whose tumors overexpress HER2 and who have
received prior therapy including an anthracycline, a taxane, and trastuzumab.
Previously marketed drugs from the 4-anilinoquinazoline class include
erlotinib (Tarceva) and gefitinib (IressaTM), both of which are indicated for
treating non-small-cell lung cancer (NSCLC). As with erlotinib and gefitinib, To Market, To Market 2007 475 lapatinib is an ATP-competitive kinase inhibitor. It inhibits the tyrosine kinase activity EGFR and HER-2 with apparent Ki values of 3 and 13 nM, respectively, and has slow off-rate kinetics (t1/2X300 min).
In addition, dividing the daily dose of lapatinib results in approximately 2-fold higher exposure at steady state compared to the same total dose administered once daily.
The chemical synthesis of lapatinib entails the condensation of 4-chloro-6-iodoquinazoline and 3-chloro-4-(3-fluorobenzyloxy)aniline to produce a diaryl amine intermediate followed by Stille coupling of the iodo group with 5-dioxolanyl-2-(tributylstannyl)furan and subsequent acid hydrolysis of the cyclic ketal to the corresponding aldehyde. Finally, reductive amination of the aldehyde intermediate with 2-(methanesulfonyl) ethylamine in the presence of sodium triacetoxyborohydride produces lapatinib.
.
Originator
GSK (US)
The Uses of Lapatinib
Lapatinib Ditosylate (GW572016, GW2016, Tykerb, Tyverb) is a potent EGFR and ErbB2 inhibitor with IC50 of 10.8 and 9.2 nM, respectively.
The Uses of Lapatinib
Lapatinib, used in the form of Lapatinib Ditosylate, is a potent EGFR and ErbB2 inhibitor with IC50 of 10.8 and 9.2 nM, respectively. It is a antineoplastic which is used in breast cancer research and a tyrosine kinase inhibitor. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2)
Background
Lapatinib is an anti-cancer drug developed by GlaxoSmithKline (GSK) as a treatment for solid tumours such as breast and lung cancer. It was approved by the FDA on March 13, 2007, for use in patients with advanced metastatic breast cancer in conjunction with the chemotherapy drug capecitabine. Lapatinib is a human epidermal growth factor receptor type 2 (HER2/ERBB2) and epidermal growth factor receptor (HER1/EGFR/ERBB1) tyrosine kinases inhibitor. It binds to the intracellular phosphorylation domain to prevent receptor autophosphorylation upon ligand binding.
Indications
Indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress the human epidermal receptor type 2 (HER2) protein and who have received prior therapy including an anthracycline, a taxane, and trastuzumab.
What are the applications of Application
Lapatinib is an antineoplastic agent used in breast cancer research
Definition
ChEBI: Lapatinib is an organofluorine compound, an organochlorine compound, a member of quinazolines and a member of furans. It has a role as an antineoplastic agent and a tyrosine kinase inhibitor. It is functionally related to a monofluorobenzene.
brand name
Tykerb
General Description
Class: receptor tyrosine kinase
Treatment: HER2-positive breast cancer
Elimination half-life = 24 h
Protein binding = 99%
Pharmacokinetics
Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. An anti-cancer drug, lapatinib was developed by GlaxoSmithKline (GSK) as a treatment for solid tumours such as breast and lung cancer. It was approved by the FDA on March 13, 2007, for use in patients with advanced metastatic breast cancer in conjunction with the chemotherapy drug capecitabine.
Metabolism
Lapatinib undergoes extensive metabolism, primarily by CYP3A4 and CYP3A5, with minor contributions from CYP2C19 and CYP2C8 to a variety of oxidated metabolites, none of which accounts for more than 14% of the dose recovered in the feces or 10% of lapatinib concentration in plasma.
Storage
Store at -20°C
References
1) Wood?et al. (2004),?A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells;? Cancer Res.,?64?6652 2) Burris?et al. (2004),?Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib;? Oncologist,?9?10 3) Chu?et al. (2005),?The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016) cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer;? Cancer Res.,?65?18
Properties of Lapatinib
| Melting point: | 144-146oC |
| Boiling point: | 750.7±60.0 °C(Predicted) |
| Density | 1.381±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Soluble in DMSO (up to 200 mg/ml) |
| form | solid |
| pka | 6.34±0.19(Predicted) |
| color | Yellow |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 231277-92-2(CAS DataBase Reference) |
Safety information for Lapatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H362:Reproductive toxicity, effects on or via lactation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lapatinib
| InChIKey | BCFGMOOMADDAQU-UHFFFAOYSA-N |
| SMILES | N1=C2C(C=C(C3=CC=C(CNCCS(C)(=O)=O)O3)C=C2)=C(NC2=CC=C(OCC3=CC=CC(F)=C3)C(Cl)=C2)N=C1 |
Lapatinib manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Lapatinib 98%View Details
Lapatinib 98%View Details
231277-92-2 -
 Lapatinib 231277-92-2 98%View Details
Lapatinib 231277-92-2 98%View Details
231277-92-2 -
 231277-92-2 Lapatinib 98%View Details
231277-92-2 Lapatinib 98%View Details
231277-92-2 -
 231277-92-2 98%View Details
231277-92-2 98%View Details
231277-92-2 -
 231277-92-2 Lapatinib 99%View Details
231277-92-2 Lapatinib 99%View Details
231277-92-2 -
 Lapatinib 98% (HPLC) CAS 231277-92-2View Details
Lapatinib 98% (HPLC) CAS 231277-92-2View Details
231277-92-2 -
 Lapatinib 98% CAS 231277-92-2View Details
Lapatinib 98% CAS 231277-92-2View Details
231277-92-2 -
 Lapatinib CAS 231277-92-2View Details
Lapatinib CAS 231277-92-2View Details
231277-92-2
