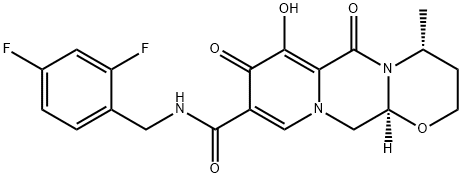
4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
- CAS NO.:500287-72-9
- Empirical Formula: C22H18N6
- Molecular Weight: 366.42
- MDL number: MFCD11046372
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile Structural](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)
What is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile?
Absorption
Rilpivirine has a Tmax of 3-4 hours and has a mean AUC of 2235 ± 851 ng*h/mL. A 25mg dose reaches a Cmax of 247 ng/mL in healthy subjects and 138.6 ng/mL in patients with HIV-1.
Toxicity
In the event of an overdose, contact a poison control centre. Patients should be treated with symptomatic and supportive measures, including monitoring of the QT interval. Dialysis is not expected to remove significant amounts of the drug from plasma as it is highly bound to albumin.
Description
In May 2011, the U.S. FDA approved rilpivirine in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV) 1 infection in treatment-naive adult patients. Rilpivirine is a member of the nonnucleoside reverse transcriptase inhibitor (NNRTI) class of anti-HIV agents. It is highly potent against a range of wild-type HIV strains (EC50=0.07–1.0 nM),~10–20 timesmore potent than the NNRTI efavirenz (Sustiva), and active against HIV strains resistant to other NNRTIs. The discovery of rilpivirine was guided by molecular modeling and X-ray crystallography of HIV-1 RT complexed with inhibitors. The synthesis of rilpivirine is accomplished by an efficient 6-step route in which the key step is coupling of 4-((4-chloropyrimidin-2-yl)amino)benzonitrile with (E)-3-(4-amino-3,5-dimethylphenyl)acrylonitrile.
Chemical properties
N/ABright Yellow Solid
Originator
Janssen (Belgium)
The Uses of 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
A novel non-nucleoside reverse transcriptase inhibitor. Rilpivirine seems to be well tolerated with less CNS disturbance than Efavirenz, and has non-teratogenic potential.
The Uses of 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
A novel non-nucleoside reverse transcriptase inhibitor. Rilpivirine seems to be well tolerated with less CNS disturbance than Efavirenz, and has non-teratogenic potential. An anti-HIV agent.
Background
Rilpivirine is non-nucleoside reverse transcriptase inhibitor (NNRTI) which is used for the treatment of HIV-1 infections in treatment-naive patients. It is a diarylpyrimidine derivative. The internal conformational flexibility of rilpivirine and the plasticity of it interacting binding site gives it a very high potency and reduces the chance of resistance compared to other NNRTI's. Rilpivirine was developed by Tilbotec, Inc. and FDA approved on May 20, 2011. On November 21, 2017, Rilpivirine, in combination with dolutegravir, was approved as part of the first complete treatment regimen with only two drugs for the treatment of adults with HIV-1 named Juluca. Rilpivirine in combination with cabotegravir was granted FDA approval on 21 January 2021. While previously administered once-monthly only, this combination product was granted FDA approval for dosing every two months on February 01, 2022 and without the need for an oral lead-in period prior.
Indications
Rilpivirine, in combination with other agents, is indicated for the treatment of HIV-1 infections in antiretroviral treatment-naive patients with HIV-1 RNA ≤100,000 copies/mL and CD4+ cell count >200 cells/mm3. The FDA combination therapy approval of rilpivirine and dolutegravir is indicated for adults and adolescents 12 years of age and older weighing at least 35 kg with HIV-1 infections whose virus is currently suppressed (< 50 copies/ml) on a stable regimen for at least six months, without a history of treatment failure and no known substitutions associated to resistance to any of the two components of the therapy.
Rilpivirine in combination with cabotegravir is indicated as a complete regimen for the treatment of HIV-1 infection in adults and adolescents - ≥12 years old and weighing at least 35kg - to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.
Definition
ChEBI: An aminopyrimidine that is pyrimidine-2,4-diamine in which the amino groups at positions 2 and 4 are substituted by 4-cyanophenyl and 4-[(E)-2-cyanovinyl]-2,6-dimethylphenyl groups respectively. Used for treatment of HIV.
brand name
Edurant
Pharmacokinetics
Rilpivirine is a non-nucleoside reverse transcriptase inhibitor that inhibits the replication of HIV-1. It has a long duration of action as the oral tablet is given daily and the intramuscular suspension is given monthly. Patients should be counselled regarding the risk of hypersensitivity reactions, hepatotoxicity, depressive disorders, and the redistribution or accumulation of body fat.
Clinical Use
Non-nucleoside reverse transcriptase inhibitor:
Treatment of progressive or advanced HIV infection
in combination with at least two other antivirals
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with clarithromycin and
erythromycin - concentration possibly increased;
concentration decreased by rifampicin and rifabutin
- avoid with rifampicin, increase dose of rilpivirine
to 50 mg daily.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, oxcarbazepine,
phenobarbital, primidone and phenytoin - avoid.
Corticosteroids: avoid with dexamethasone (except
as a single dose).
Orlistat: absorption possibly reduced by orlistat.
Ulcer-healing drugs: concentration possibly reduced
by esomeprazole, lansoprazole, omeprazole,
pantoprazole and rabeprazole - avoid; avoid
histamine H2
-antagonists for 12 hours before and 4
hours after rilpiverine.
Metabolism
Rilpivirine is predominantly metabolized by CYP3A4 and CYP3A5 to the hydroxylated metabolites M1, M2, M3, and M4. UGT1A1 glucuronidates the M2 metabolite to form M6, UGT1A4 glucuronidates rilpivirine to form M5, and an unknown UGT glucuronidates the M4 metabolite to form M7.
Metabolism
Primarily undergoes oxidative metabolism mediated by
the cytochrome P450 (CYP) 3A system.
85% excreted via the faeces (25% as unchanged drug) and
6% via the urine.
storage
Store at -20°C
References
[1] moss d m, liptrott n j, curley p, et al. rilpivirine inhibits drug transporters abcb1, slc22a1, and slc22a2 in vitro. antimicrobial agents and chemotherapy, 2013, 57(11): 5612-5618.
[2] garvey l, winston a. rilpivirine: a novel non-nucleoside reverse transcriptase inhibitor. 2009.
[3] weiss j, haefeli w e. potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. international journal of antimicrobial agents, 2013, 41(5): 484-487.
[4] baert l, van’t klooster g, dries w, et al. development of a long-acting injectable formulation with nanoparticles of rilpivirine (tmc278) for hiv treatment. european journal of pharmaceutics and biopharmaceutics, 2009, 72(3): 502-508.
Properties of 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
| Melting point: | 245℃ |
| Boiling point: | 634.1±65.0 °C(Predicted) |
| Density | 1.27 |
| storage temp. | Refrigerator |
| solubility | Acetone (Slightly), Chloroform (Slightly), DMSO (Slightly), Water (Very Slightly |
| form | Solid |
| pka | 4.56±0.10(Predicted) |
| color | Yellow |
Safety information for 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
Computed Descriptors for 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 500287-72-9 Rilpivirine 98%View Details
500287-72-9 Rilpivirine 98%View Details
500287-72-9 -
 500287-72-9 98%View Details
500287-72-9 98%View Details
500287-72-9 -
 Rilpivirine 98%View Details
Rilpivirine 98%View Details
500287-72-9 -
 Rilpivirine 98% (HPLC) CAS 500287-72-9View Details
Rilpivirine 98% (HPLC) CAS 500287-72-9View Details
500287-72-9 -
 Rilpivirine CAS 500287-72-9View Details
Rilpivirine CAS 500287-72-9View Details
500287-72-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1