Cobalt chloride
Synonym(s):Cobaltous chloride;Dichlorocobalt;Cobalt dichloride;Cobalt muriate;Cobalt(II)-chloride solution
- CAS NO.:7646-79-9
- Empirical Formula: Cl2Co
- Molecular Weight: 129.84
- MDL number: MFCD00010938
- EINECS: 231-589-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:08
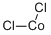
What is Cobalt chloride?
Description
blue crystals (anhydrous)
violet-blue (dihydrate)
rose red crystals (hexahydrate)
Sinks and mixes with water. Pale blue leaflets, turns pink upon exposure to moist air.
Chemical properties
(1) Blue, (2) ruby-red crystals.Soluble in water, alcohol, and acetone.
Physical properties
Blue leaflets; turns pink in moist air; hygroscopic; the dihydrate is violet blue crystal; the hexahydrate is pink monoclinic crystal; density 3.36, 2.48 and 1.92 g/cm3 for anhydrous salt, dihydrate and hexahydrate, respectively; anhydrous salt melts at 740°C and vaporizes at 1,049°C; vapor pressure 60 torr at 801°C; the hexahydrate decomposes at 87°C; the anhydrous salt and the hydrates are all soluble in water, ethanol, acetone, and ether; the solubility of hydrates in water is greater than the anhydrous salt.
The Uses of Cobalt chloride
Cobalt(II) chloride is used in humidity indicator in weather instruments. In the anhydrous form, it finds use in electroplating of cobalt, in organic chemistry and is a precursor to cobaltocene, (bis(cyclopentadienyl)cobalt(II), which is a good reducing agent. It also serves as a Lewis acid. Cobalt chloride is an indicator for water in desiccants, owing to the reversible hydration/dehydration coupled with distinct color change. Cobalt chloride is useful for producing invisible ink as it turns blue when heated and becomes invisible once it gets cooled. Cobalt(II) chloride catalyzes cross coupling of aryl halides or vinyl halides with aryl grignard reagents in excellent yields.
The Uses of Cobalt chloride
Absorbent for ammonia, gas masks, electroplating, sympathetic inks, hygrometers, manufacture of vitamin B 12, flux for magnesium refining, solid lubricant, dye mordant, catalyst, barometers, laboratory reagent, fertilizer additive.
The Uses of Cobalt chloride
Cobalt chloride (CoCl2) is used to manufacture vitamin B12, even though the compound itself can cause damage to red blood cells. It is also used as a dye mordant (to fix the dye to the textile so that it will not run). It is also of use in manufacturing solid lubricants, as an additive to fertilizers, as a chemical reagent in laboratories, and as an absorbent in gas masks, electroplating, and the manufacture of vitamin B12.
What are the applications of Application
Cobalt(II) chloride is the most common cobalt compound found in laboratories used as a moisture indicator
Definition
ChEBI: A cobalt salt in which the cobalt metal is in the +2 oxidation state and the counter-anion is chloride. It is used as an indicator for water in desiccants.
General Description
Cobalt(II) chloride is an anhydrous cobalt salt. Cobalt(II) chloride participates in the synthesis of various esters in the presence of acetonitrile.
Air & Water Reactions
Hygroscopic. Soluble in water.
Reactivity Profile
A 0.2 molar aqueous solution has a pH of 4.6. Cobalt chloride acts as a weakly acidic inorganic salt, which is soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Potassium or sodium metals act to reduce metal halides, producing exothermic reactions, even explosions [Bretherick, 5th Ed., 1995].
Hazard
May not be used in food products (FDA). Can cause blood damage.
Health Hazard
Inhalation causes respiratory disease, shortness of breath, and coughing; permanent disability may occur. Ingestion causes pain, vomiting, and diarrhea. Contact causes irritation of eyes and may cause skin rash.
Fire Hazard
Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Cobalt chloride induces hypoxia condition in cells by upregulating hypoxia-inducible factor-1α (HIF-1α), erythropoietin and glycolytic enzymes. It is also responsible for mitochondrial DNA damage in rat neuronal PC12 cells. It is also responsible for the induction of apoptosis.Cobalt chloride 0.1M solution is an additive screening solution of Additive Screening Kit. Additive Screen kit is designed to allow rapid and convenient evaluation of additives and their ability to influence the crystallization of the sample. The Additive Kit provides a tool for refining crystallization conditions.
Safety Profile
Suspected carcinogen with experimental carcinogenic data. Poison experimentally by ingestion, skin contact, intraperitoneal, intravenous, and subcutaneous routes. Moderately toxic to humans by ingestion. Human systemic effects by ingestion: anorexia, goiter (increased thyroid size), and weight loss. Experimental teratogenic and reproductive effects. Human mutation data reported. Incompatible with metals (e.g., sodmm and potassium). See also COBALT. When heated to decomposition it emits toxic fumes of Cl-.
Metabolism
Not Available
Purification Methods
A saturated aqueous solution at room temperature is fractionally crystallised by standing overnight. The first half of the material that crystallises in this way is used in the next crystallisation. The process is repeated several times, water being removed in a dry-box using air filtered through glass wool and dried over CaCl2 [Hutchinson J Am Chem Soc 76 1022 1954]. It has also been crystallised from dilute aqueous HCl. The hexahydrate m 86o forms pink to red deliquescent crystals. It loses 4H2O on heating at 52-56o and forms the violet dihydrate which loses a further H2O at 100o to form the violet monohydrate which loses the last H2O at 120-140o to give the pale blue anhydrous deliquescent salt m 735o and b 1049o. A pink solution of CoCl2 in H2O becomes blue on heating to 50o or adding conc HCl which may precipitate the mono or dihydrate. The solid dihydrate gives a blue-purple solution with EtOH. Note: CoCl2 in H2O is a “sympathetic ink”, i.e. writing using an aqueous solution is almost invisible on paper, but becomes blue on warming the paper. On cooling or standing, the writing becomes invisible again. The anhydrous salt is soluble in H2O, EtOH, Et2O, Me2CO and pyridine. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1515 1965.]
Properties of Cobalt chloride
| Melting point: | 724 °C(lit.) |
| Boiling point: | 1049 °C |
| Density | 3.35 |
| vapor pressure | 40 mm Hg ( 0 °C) |
| Flash point: | 500°C |
| storage temp. | Store below +30°C. |
| solubility | 585.9g/l soluble |
| form | beads |
| color | Pale blue |
| Specific Gravity | 3.356 |
| PH | pH (50g/l, 25℃) : >=3.0 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Sublimation | 500 ºC |
| Merck | 14,2437 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7646-79-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Cobalt dichloride(7646-79-9) |
| EPA Substance Registry System | Cobalt chloride (CoCl2) (7646-79-9) |
Safety information for Cobalt chloride
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobalt chloride
Cobalt chloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Cobalt chloride 98%View Details
Cobalt chloride 98%View Details -
 Cobalt chloride 99%View Details
Cobalt chloride 99%View Details -
 Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
7646-79-9 -
 Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
7646-79-9 -
 Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
7646-79-9 -
 Cobalt(II) chloride, ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, ultra dry CAS 7646-79-9View Details
7646-79-9 -
 Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
7646-79-9 -
 Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
Cobalt(II) chloride, Ultra dry CAS 7646-79-9View Details
7646-79-9
