Clevudine
- CAS NO.:163252-36-6
- Empirical Formula: C10H13FN2O5
- Molecular Weight: 260.22
- MDL number: MFCD00935785
- EINECS: 200-001-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
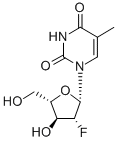
What is Clevudine?
Description
Clevudine is a fluorinated b-L-nucleoside analog launched for the
oral treatment of chronic HBV infection. It is the fifth nucleoside or nucleotide
analog to be marketed for this indication. The previous drugs from this class
include lamivudine, adefovir, entecavir, and telbivudine. In HBV-expressing
human hepatoma cell line 2.2.15, clevudine inhibits HBV DNA synthesis with
an EC50 of 0.1 μM, and does not show cytotoxicity up to 200 μM. It is
phosphorylated by cellular kinases to the active triphosphate derivative,
which subsequently inhibits HBV DNA polymerase and HBV replication.
466 Shridhar Hegde and Michelle Schmidt
Clevudine-5′-triphosphate has an intracellular half-life of 16.5 h. Interestingly, it
is a non-competitive inhibitor of viral polymerase, and inhibits HBV replication
without being incorporated into the DNA. The pharmacokinetic profile of clevudine was linear with a plasma half-life of approximately 60 h. Clevudine was undetectable in plasma after 4 weeks following the cessation of dosing.The most common adverse events reported with clevudine treatment include infection, asthenia, dyspepsia, abdominal pain, headache, and diarrhea.
Clevudine is chemically derived from L-ribose by first incorporating acyl protective groups to produce 1-O-acetyl-2,3,5-tri-O-benzoyl-b-L-ribofuranose intermediate, which is then converted to 1,3,5-tri-O-benzoyl-a-L-ribofuranose in two steps by treating To Market, To Market 2007 467 with hydrogen chloride and subsequent hydrolysis and acyl migration. The remaining steps leading to clevudine include conversion of the C2-hydroxy group to C2-fluoro group with triethylamine trihydrofluoride, formation of the corresponding ribofuranosyl bromide intermediate with hydrogen bromide and acetic acid, condensation with silylated thymine, and removal of benzoyl protective groups with methanolic ammonia.
Chemical properties
White Solid
Originator
University of Georgia/ Yale University (US)
The Uses of Clevudine
Antiviral drug, used for the treatment of Hepatitis B.
Definition
ChEBI: Clevudine is a pyrimidine 2'-deoxyribonucleoside.
brand name
Levovir
Synthesis
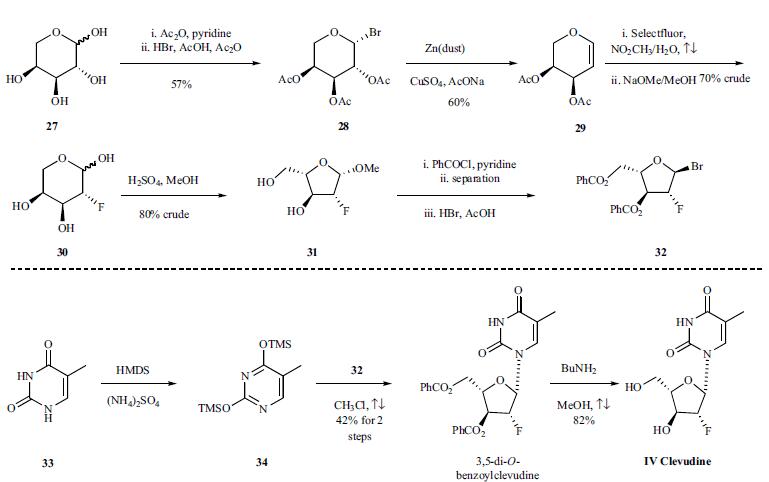
The synthesis is depicted in the scheme. L-Arabinose (27) was treated with acetic anhydride and pyridine at room temperature for four hours to give acetylated arabinose, which was then brominated using 30% HBr in AcOH/Ac2O at room temperature for 36 hours to afford bromo-sugar 28 as a white solid in 57% yield after crystallization in ethyl ether. Bromo-sugar 28 was then treated with Zn dust, CuSO4 and NaOAc in AcOH/H2O, followed by chromatographic separation to give L-arabinal 29 in 60% yield. L-arabinal 29 was converted to the fluoro derivative in 70% crude yield by reaction with Selectfluor® (FTEDA- BF4) in refluxing nitromethane/H2O, and the resulting fluoroalcohol was deacetylated with NaOMe in MeOH to give compound 30 in 100% crude yield. Compound 30 was then treated with H2SO4 in refluxing MeOH to afford methyl furanoside 31 in 80% crude yield. Furanoside 31 was benzoylated with benzoyl chloride in pyridine to give a mixture of isomers, from which the |á-anomer was isolated by chromatography and then brominated with 30% HBr/AcOH in CH2Cl2 to provide the crude bromo-sugar 32 which was dissolved in chloroform and used without further purification in the next step. Compound 34 was obtained by treatment of thymine (33) with HMDS and ammonium sulfate in refluxing chloroform for 16 hours. The sugar 32 was condensed with silylated pyrimidine derivative 34 in refluxing chloroform to afford 3,5-di-O-benzoylclevudine in 42% yield after recrystallization from ethanol. The benzoyl groups were removed upon treatment with n-butylamine in refluxing methanol to give clevudine (IV) in 82% yield.
Properties of Clevudine
| Melting point: | 184-185° |
| alpha | D25 -111.77° (c = 0.23 in methanol) |
| Density | 1.55±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 9.55±0.10(Predicted) |
| form | Solid |
| color | White |
| CAS DataBase Reference | 163252-36-6 |
Safety information for Clevudine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Clevudine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Clevudine 95% CAS 163252-36-6View Details
Clevudine 95% CAS 163252-36-6View Details
163252-36-6 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4