Adefovir dipivoxil
Synonym(s):9-(2[bis(Pivaloyloxymethoxy)phosphorylmethoxy]ethyl)adenine
- CAS NO.:142340-99-6
- Empirical Formula: C20H32N5O8P
- Molecular Weight: 501.47
- MDL number: MFCD00869897
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
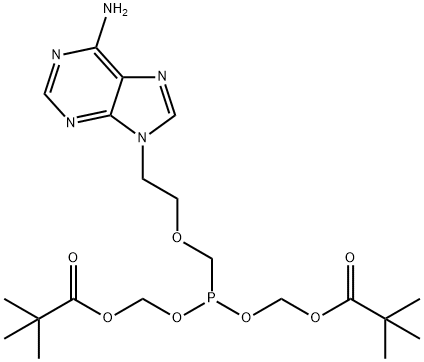
What is Adefovir dipivoxil?
Absorption
The approximate oral bioavailability of adefovir from HEPSERA is 59%. When a single oral 10 mg dose is given to chronic hepatitis B patients, the peak plasma concentration (Cmax) of adefovir was 18.4 ± 6.26 ng/mL. This occurred between 0.58 - 4 hours post dose (Tmax). The adefovir area under the plasma concentration-time curve (AUC0–∞) was 220 ± 70.0 ng?h/mL. Food does not affect the exposure of adeforvir.
Toxicity
Renal tubular nephropathy characterized by histological alterations and/or increases in BUN and serum creatinine was the primary dose-limiting toxicity associated with administration of adefovir dipivoxil in animals. Nephrotoxicity was observed in animals at systemic exposures approximately 3–10 times higher than those in humans at the recommended therapeutic dose of 10 mg/day.
Description
Adefovir dipivoxil is the first nucleotide analog to be launched in the US as an oral treatment for hepatitis B virus (HBV) infections. It can be easily prepared in 4 steps from adenine. Adefovir dipivoxil acts as a bioavailable ester prodrug which is rapidly hydrolyzed to free adefovir and further anabolized to its active form, adefovir diphosphate, by two intracellular phosphotylation steps. The diphosphate competitively inhibits reverse transcriptase and/or causes chain termination when incorporated into growing DNA. Adefovir dipivoxil has a broad antiviral spectrum against retro-, herpes- and hepadnaviruses. The drug inhibits HBV replication, decreases HBV DNA levels and improves liver histology of patients infected with HBV wild type and resistant to other antivirals such as lamivudine. It also demonstrated activity in hepatitis B”e” antigennegative, or precore mutant, patients and in patients co-infected with HIV. To date, no adefovir dipivoxil-associated resistance mutations have been identified in patients up to 136 weeks with the drug. The oral bioavailability of adefovir after oral administration of its dipivoxil prodrug is approximately 30%. It is mainly excreted unchanged in the urine and its plasma elimination half-life is 4.2 h. However, a long intracellular half-life (17 h) of the active bisphosphorylated metabolite enables once-daily dosing. The most prominent adverse effect of adefovir dipivoxil is nephrotoxicity (which has prevented the drug from being marketed for HIV infections where the drug required administration at higher doses).
Chemical properties
White Solid
Originator
Institute of Organic Chemistry and Biochemistry of the Academy of Sciences in the Czech Republic and the REGA Stichting Research (Czech Republic, Belgium)
The Uses of Adefovir dipivoxil
Adefovir Dipivoxil(Preveon, Hepsera) works by blocking reverse transcriptase, an enzyme that is crucial for the hepatitis B virus (HBV) to reproduce in the body. It is approved for the treatment of chronic hepatitis B in adults with evidence of active vir
The Uses of Adefovir dipivoxil
A nucleotide analog, useful as an oral reverse transcriptase inhibitor (ntRTI).
What are the applications of Application
Adefovir dipivoxil is an antiviral acyclic nucleoside phosphonate (ANP) analog
Background
Adefovir dipivoxil, previously called bis-POM PMEA, with trade names Preveon and Hepsera, is an orally-administered acyclic nucleotide analog reverse transcriptase inhibitor (ntRTI) used for treatment of hepatitis B. It is ineffective against HIV-1. Adefovir dipivoxil is the diester prodrug of adefovir.
Indications
Indicated for the treatment of chronic hepatitis B in adult patients with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease; this is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and in adult patients with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function.
Definition
ChEBI: Adefovir pivoxil is an organic phosphonate that is the dipivoxil ester of adefovir. A prodrug for adefovir, an HIV-1 reverse transcriptase inhibitor, adefovir pivoxil is used to treat chronic hepatitis B viral infection. It has a role as a prodrug, an antiviral drug, a DNA synthesis inhibitor, a HIV-1 reverse transcriptase inhibitor and a nephrotoxic agent. It is an organic phosphonate, a member of 6-aminopurines, an ether and a carbonate ester. It is functionally related to an adefovir.
brand name
Hepsera (Gilead Sciences).
General Description
Adefovir is an orally active prodrug that is indicated for thetreatment of the chronic form of hepatitis B. The dipivoxil moieties are hydrolyzed by ubiquitous esterases to yieldadefovir, which is phosphorylated by adenylate kinase toyield adefovir diphosphate. This compound is inhibitory atHBV DNA polymerase. In addition, adefovir undergoes incorporationinto viral DNA and causes chain termination.Adefovir is poorly absorbed by the oral route, but the dipivoxilester groups cause the bioavailability to increase toapproximately 60%.
Mechanism of action
Adefovir dipivoxil is an orally active prodrug indicated for the treatment of chronic hepatitis B. The drug is hydrolyzed by extracellular esterases to produce adefovir, which in turn is phosphorylated by adenylate kinase to adefovir diphosphate, which inhibits HBV DNA polymerase. Incorporation of adefovir into viral DNA also leads to DNA chain termination. As shown in Figure 43.9, adefovir dipivoxyl is activated in two steps involving an esterase that exposes a free phosphate group (adefovir), followed by addition of a second phosphate by adenylate kinase to form adefovir diphosphate, the active form of the drug.
Pharmacokinetics
Adefovir dipivoxil a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV). The concentration of adefovir that inhibited 50% of viral DNA synthesis (IC50) in vitro ranged from 0.2 to 2.5 μM in HBV transfected human hepatoma cell lines. The combination of adefovir with lamivudine showed additive anti-HBV activity.
Clinical Use
Adefovir dipivoxil joins interferon and lamivudine in the treatment of chronic HBV. It can be used singly or in combination with lamivudine. Early clinical studies indicate benefit of the use of adefovir dipivoxil to treat lamivudine-resistant HBV with a low level of resistant virus developing to monotherapy with adefovir dipivoxil.
Synthesis
Steroidal dutasteride (5) was synthesized from 3-oxo-4-
androstene-17|?-carboxylic acid (55). Oxidation of 55
with potassium permanganate, sodium periodate and sodium
carbonate in refluxing t-butyl alcohol and water gave secosteroid
56 which was cyclized with ammonium acetate in
acetic acid to give 4-aza-steroid 57 in good yield. Stereoselective
hydrogenation of 57 with H2 over PtO2 in hot
acetic acid and in the presence of ammonium acetate yielded
saturated azasteroid 58, which was dehydrogenated with
DDQ in the presence of bis(trimethylsilyl)trifluoroacetamide
(BSTFA) 59 in refluxing dioxane to give 60. Treatment of
60 with thionyl chloride gave the corresponding acyl
chloride intermediate, which was then condensed with 2,5-
bis(trifluoromethyl)aniline (61) by means of DMAP in
heated toluene to give dutasteride (5) in 57% yield from
intermediate 60.
Drug interactions
Potentially hazardous interactions with other drugs
Use with caution in combination with other
nephrotoxins.
Antivirals: avoid concomitant administration with
tenofovir
Interferons: use with caution with peginterferon alfa.
Metabolism
Following oral administration, adefovir dipivoxil is rapidly converted to adefovir. 45% of the dose is recovered as adefovir in the urine over 24 hours at steady state following 10 mg oral doses. Adefovir is not a substrate of the cytochrome P450 enzymes.
Metabolism
Adefovir is poorly absorbed orally, but the bioavailability of adefovir dipivoxil reaches approximately 59%. The drug is absorbed to an equal extent with or without the presence of food. Adefovir is excreted renally unchanged.
Properties of Adefovir dipivoxil
| Melting point: | 98-102°C |
| Boiling point: | 641.0±65.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ethanol: soluble50mg/mL |
| form | solid |
| pka | 4.16±0.10(Predicted) |
| color | White to Off-White |
| Merck | 14,151 |
| CAS DataBase Reference | 142340-99-6(CAS DataBase Reference) |
Safety information for Adefovir dipivoxil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Adefovir dipivoxil
| InChIKey | WOZSCQDILHKSGG-UHFFFAOYSA-N |
Abamectin manufacturer
BDR Pharmaceuticals International Pvt Ltd
Aspen Biopharma Labs Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![[[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid diethyl ester](https://img.chemicalbook.in/CAS/GIF/116384-53-3.gif)


You may like
-
 142340-99-6 Adefovir Dipivoxil 98%View Details
142340-99-6 Adefovir Dipivoxil 98%View Details
142340-99-6 -
 142340-99-6 98%View Details
142340-99-6 98%View Details
142340-99-6 -
 Adefovir Dipivoxil 98%View Details
Adefovir Dipivoxil 98%View Details
142340-99-6 -
 Adefovir Dipivoxil 98%View Details
Adefovir Dipivoxil 98%View Details
142340-99-6 -
 142340-99-6 95-99 %View Details
142340-99-6 95-99 %View Details
142340-99-6 -
 Adefovir Dipivoxil CAS 142340-99-6View Details
Adefovir Dipivoxil CAS 142340-99-6View Details
142340-99-6 -
 Adefovir dipivoxil CAS 142340-99-6View Details
Adefovir dipivoxil CAS 142340-99-6View Details
142340-99-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4