Dideoxyinosine
Synonym(s):2′,3′-Dideoxyinosine;ddI;ddIno
- CAS NO.:69655-05-6
- Empirical Formula: C10H12N4O3
- Molecular Weight: 236.23
- MDL number: MFCD00077728
- EINECS: 614-994-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
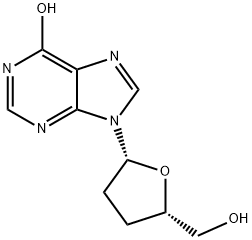
What is Dideoxyinosine?
Absorption
Rapidly absorbed (bioavailability 30-40%) with peak plasma concentrations appearing within 0.5 and 1.5 hrs.
Toxicity
Side effects include pancreatitis, peripheral neuropathy, diarrhea, hyperuricemia and hepatic dysfunction
Description
Didanosine is an orally active purine dideoxynucleoside analog indicated for adult and pediatric patients with advanced HIV infection who are either intolerant or significantly deteriorated on zidovudine. It appears to increase CD4 cell counts and decrease p24 antigen levels.Major adverse effects are pancreatitis, peripheral neuropathy and diarrhea.Unlike zidovudine, didanosine exhibits insignificant bone marrow suppression.
Chemical properties
White Powder
Originator
National Cancer Institute(NIH) (U.S.A.)
The Uses of Dideoxyinosine
2?,3?-Dideoxyinosine is a potent anti-retroviral agent. It is most effective in combination therapy for the treatment of HIV and related lymphoma.
The Uses of Dideoxyinosine
Used as an antiviral
The Uses of Dideoxyinosine
Antiviral;Transrcriptase inverse inhibitor
Indications
For use, in combination with other antiretroviral agents, in the treatment of HIV-1 infection in adults.
Background
A dideoxynucleoside compound in which the 3'-hydroxy group on the sugar moiety has been replaced by a hydrogen. This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. Didanosine is a potent inhibitor of HIV replication, acting as a chain-terminator of viral DNA by binding to reverse transcriptase; ddI is then metabolized to dideoxyadenosine triphosphate, its putative active metabolite.
What are the applications of Application
2′,3′-Dideoxyinosine is a potent anti-retroviral agent that acts by competing with natural dATP
Definition
ChEBI: A purine 2',3'-dideoxyribonucleoside that is inosine in which the hydroxy groups at both the 2' and the 3' positions on the sugar moiety have been replaced by hydrogen.
Indications
Didanosine (ddI, Videx) is an adenosine analogue with activity against HIV-1, HIV-2, and HTLV-I. It is approved as part of a multidrug regimen for the therapy of HIV infection and is also used as postexposure HIV prophylaxis
Manufacturing Process
In a 500 ml flask with a shoulder was separately charged 50 ml of medium
(pH 7.0) containing 0.5 g/dl of yeast extract, 1.0 g/dl of peptone, 1.0 g/dl of
meat extract and 0.5 g/dl of NaCl followed by sterilization. One platinum loop
of each microorganism shown in Table which had been preincubated in
bouillon agar medium at 30°C for 16 hours was inoculated on the medium
followed by shake culture at 30°C for 16 hours. After the cells were isolated
from the obtained culture solution by centrifugal separation, the cells were
washed with 0.05 M Tris-HCl buffer (pH 7.2) and further centrifuged to give
washed cells.
The washed cells described above were added to 0.05 M Tris-HCl buffer (pH
7.2) containing 1 g/dl of 2',3'-dideoxyadenosine in a concentration of 5 g/dl
followed by reacting at 30°C for 2 hours. The amount of 2',3'-dideoxyinosine
produced at this stage is shown in the next Table.
This way 2',3'-dideoxyinosine may be produced from 2',3'-dideoxyadenosine in
a short period of time, by contacting microorganisms supplied at low cost,
products containing the same or treated products thereof, with a substrate.
brand name
Videx (Bristol-Myers Squibb).
Therapeutic Function
Antiviral
Antimicrobial activity
Didanosine is active against HIV-1, HIV-2 and HTLV-1.
Acquired resistance
Codon changes at positions 65 or 74 in HIV reverse transcriptase are associated with reduced susceptibility.
General Description
Fluffy white solid or powder. Condenses at 347°F and darkens at approximately 572°F. Odorless.
General Description
Didanosine (Videx, ddI) is 2',3'-dideoxyinosine (ddI), a synthetic purine nucleoside analog that is bioactivatedto 2',3'-dideoxy-ATP (ddATP) by host cellularenzymes.The metabolite, ddATP, accumulates intracellularly,where it inhibits RT and is incorporated intoviral DNA to cause chain termination in HIVinfectedcells. The potency of didanosine is 10-to 100-foldless than that of AZT with respect to antiviral activity andcytotoxicity, but the drug causes less myelosuppressionthan AZT causes.
Didanosine is recommended for the treatment of patientswith advanced HIV infection who have received prolongedtreatment with AZT but have become intolerant to, or experiencedimmunosuppression from, the drug. AZT and ddIact synergistically to inhibit HIV replication in vitro, andddI is effective against some AZT-resistant strains of HIV.Painful peripheral neuropathy (tingling, numbness, and painin the hands and feet) and pancreatitis (nausea, abdominalpain, elevated amylase) are the major dose-limiting toxicitiesof didanosine. Didanosine is given orally in the form ofbuffered chewable tablets or as a solution prepared from thepowder. Both oral dosage forms are buffered to preventacidic decomposition of ddI to hypoxanthine in the stomach.
Air & Water Reactions
Water soluble.
Health Hazard
SYMPTOMS: Symptoms of exposure to a related compound include cutaneous eruptions, fever, mouth sores, thrombocytopenia, neutropenia, reversible peripheral neuropathy, gastrointestinal distress, headache, nausea and vomiting.
Fire Hazard
Flash point data for Dideoxyinosine are not available; however, Dideoxyinosine is probably combustible.
Pharmaceutical Applications
An analog of deoxyadenosine, formulated for oral administration.
Mechanism of action
Didanosine (ddl) is a purine dideoxynucleoside, which is an analogue of inosine. Chemically, it is 2′,3′-dideoxyinosine, and it differs from inosine by having hydrogen atoms in place of the 2′- and 3′-hydroxyl groups on the ribose ring. Didanosine is a pro-drug that is bioactivated by metabolism to dideoxyadenosine triphosphate, which is a competitive inhibitor of viral RT and is incorporated into the developing viral DNA in place of deoxyladenosine triphosphate. As such, this agent causes chain termination because of the absence of a 3′-hydroxyl group. Didanosine inhibits HIV RT and exerts a virustatic effect on the retroviruses. Combined with ZDV, antiretroviral activity of ddI is increased.
Pharmacokinetics
Didanosine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Didanosine is a hypoxanthine attached to the sugar ring, unlike other nucleoside analogues. Didanosine is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. Didanosine is effective against HIV, and usually used in combination with other antiviral therapy. Switching from long term AZT treatment to didanosine has been shown to be beneficial. Didanosine has weak acid stability and therefore, it is often combined with an antacid.
Pharmacokinetics
Oral absorption: c. 40%
Cmax 400 mg once daily: 0.93 mg/L
Plasma half-life: c. 1.4 h
Volume of distribution: c. 1 L/kg
Plasma protein binding: <5%
Absorption
Bioavailability is reduced by about half when taken with food and the drug should be given at least 30 min before a meal. The peak plasma concentration achieved by enteric-coated tablets is less than half that of buffered tablets.
Distribution
Central nervous system (CNS) penetration is relatively poor. Median concentrations in semen (455 ng/mL; range < 50–2190 ng/mL) are greater than those in blood (<50 ng/mL; range <50–860 ng/mL). It is secreted in breast milk.
Metabolism
Based upon animal studies it is presumed that metabolism occurs by the pathways responsible for the elimination of endogenous purines by xanthine oxidase. Metabolism may be altered in patients with severe hepatic impairment; however, no specific dose adjustment is recommended.
Excretion
Renal clearance by glomerular filtration and active tubular secretion accounts for 50% of total body clearance. Urinary recovery accounts for about 20% of the oral dose in adults. The half-life increases three-fold in patients requiring dialysis. Patients with a creatinine clearance <60 mL/min may be at greater risk of toxicity.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Clinical Use
The most common adverse effect produced by didanosine is diarrhea.Abdominal pain, nausea, vomiting, anorexia, and dose-related peripheral neuropathy may occur. Pancreatitis occurs rarely, as do hyperuricemia, bone marrow suppression, retinal depigmentation, and optical neuritis. Resistance to didanosine appears to result from mutations different from those responsible for zidovudine resistance.
Side Effects
Most serious are pancreatitis (fatal and non-fatal), lactic
acidosis
and severe hepatomegaly with steatosis (fatal and nonfatal),
retinopathy, optic neuritis and dose-related peripheral
neuropathy. Patients with low body weight may require dose
modification. A strong association with non-cirrhotic
portal
hypertension has been described.
The combination with stavudine should be avoided in
pregnant women as fatal cases of lactic acidosis have been
reported. Caution should also be exercised in patients
with known risk factors for liver disease. Therapy should
be stopped in patients who develop clinical or laboratory
evidence of lactic acidosis or hepatotoxicity. Monitoring
lactate levels prospectively is not recommended as mild
hyperlactatemia occurs in asymptomatic patients and has a
poor positive predictive value for the development of lactic
acidosis.
Caution should be exercised in co-administering other
drugs with known neurotoxicity and in patients with a history
of neuropathy. Treatment should stop if symptoms and
signs of neuropathy are observed, but the condition is usually
reversible and patients with resolved neuropathy may be
retreated at a reduced dosage. Retinal depigmentation has
been observed in children and twice-yearly dilated retinal
examination is recommended.
Drug interactions
Potentially hazardous interactions with other drugs
Allopurinol: concentration of didanosine increased
- avoid.
Antibacterials: ciprofloxacin, tetracyclines, and other
antibiotics affected by indigestion remedies - do not
administer within 2 hours of didanosine.
Antivirals: absorption of atazanavir reduced (give
at least 2 hours before or 1 hour after didanosine
tablets); manufacturer of darunavir advises to take
didanosine 1 hour before or 2 hours after darunavir;
didanosine tablets reduce absorption of indinavir
(give at least 1 hour apart); concentration possibly
increased by ganciclovir, valganciclovir and tenofovir
- avoid with tenofovir; give didanosine and ritonavir
at least 2.5 hours apart; increased risk of side effects
with ribavirin and stavudine - avoid; concentration
reduced by tipranavir (give tipranavir and didanosine
capsules at least 2 hours apart); give didanosine 2
hours before or 4 hours after rilpivirine.
Cytotoxics: increased risk of toxicity with
hydroxycarbamide - avoid.
Orlistat: absorption of didanosine possibly reduced.
Metabolism
Rapidly metabolized intracellularly to its active moiety, 2,3-dideoxyadenosine-5-triphosphate (ddA-TP). It is then further metabolized hepatically to yield hypoxanthine, xanthine, and uric acid.
Metabolism
Didanosine is less toxic than ZDV. The CSF fluid/plasma
ratio of ddI is 0.2. Didanosine is ultimately converted to hypoxanthine, xanthine, and uric acid through the
usual metabolic pathway for purines. The latter is a nontoxic metabolic product.
Didanosine is given in advanced HIV infection, ZDV intolerance, or significant clinical/immunologic
deterioration.
Precautions
Buffering agents that are compounded with didanosineto counteract its degradation by gastric acid mayinterfere with the absorption of other drugs that requireacidity (e.g., indinavir, delavirdine, ketoconazole, fluoroquinolones,tetracyclines, dapsone). An enteric-coatedformulation (Videx EC) that dissolves in the basic pH ofthe small intestine is not susceptible to these interactions.Ganciclovir and valganciclovir can increase bloodlevels of didanosine.The use of zalcitabine with didanosineis not recommended because that combination carriesan additive risk of peripheral neuropathy.The combinationof didanosine with stavudine increases the riskof pancreatitis, hepatotoxicity, and peripheral neuropa-thy. Stavudine should not be given with didanosine topregnant women because of the increased risk of metabolicacidosis.
Properties of Dideoxyinosine
| Melting point: | 193-195 °C |
| Boiling point: | 193-195 C |
| alpha | D25 -26.3° (c = 10 in water) |
| Density | 1.2917 (rough estimate) |
| refractive index | -28 ° (C=0.34, H2O) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO or methanol |
| form | Powder |
| pka | 9.12(at 25℃) |
| color | White to Off-white |
| Water Solubility | 1-5 g/100 mL at 21 ºC |
| Merck | 14,3098 |
| BRN | 3619529 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 69655-05-6(CAS DataBase Reference) |
| IARC | 3 (Vol. 76) 2000 |
| EPA Substance Registry System | Inosine, 2',3'-dideoxy- (69655-05-6) |
Safety information for Dideoxyinosine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Dideoxyinosine
Dideoxyinosine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 2',3'-Dideoxyinosine CAS 69655-05-6View Details
2',3'-Dideoxyinosine CAS 69655-05-6View Details
69655-05-6 -
 2',3'-Dideoxyinosine CAS 69655-05-6View Details
2',3'-Dideoxyinosine CAS 69655-05-6View Details
69655-05-6 -
 2,3-Dideoxyinosine (ddI CAS 69655-05-6View Details
2,3-Dideoxyinosine (ddI CAS 69655-05-6View Details
69655-05-6 -
 Didanosine 97% (HPLC) CAS 69655-05-6View Details
Didanosine 97% (HPLC) CAS 69655-05-6View Details
69655-05-6 -
 Didanosine 95.00% CAS 69655-05-6View Details
Didanosine 95.00% CAS 69655-05-6View Details
69655-05-6 -
 Didanosine CAS 69655-05-6View Details
Didanosine CAS 69655-05-6View Details
69655-05-6 -
 69655-05-6 Didanosine 98%View Details
69655-05-6 Didanosine 98%View Details
69655-05-6 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
