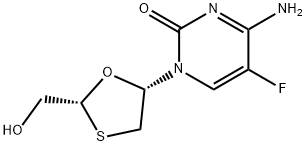
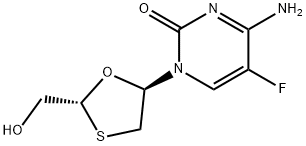
Emtricitabine
Synonym(s):(−)-2′3′-Dideoxy-5-fluoro-3′-thiacytidine;(2R,5S)-4-Amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one;FTC
- CAS NO.:143491-57-0
- Empirical Formula: C8H10FN3O3S
- Molecular Weight: 247.25
- MDL number: MFCD00870151
- EINECS: 604-363-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is Emtricitabine?
Absorption
Emtricitabine reaches a Cmax of 1.8±0.7μg/mL with a Tmax of 1-2 hours, and has an AUC of 10±3.1μg*hr/mL. The bioavailability of emtricitabine capsules is 93% and the bioavailability of the oral solution is 75%. Taking emtricitabine with food decreases the Cmax by 29%.[L9019
Toxicity
The LD50 of emtricitabine is not readily available.[9019,L9818]
Symptoms of emtricitabine toxicity include hepatotoxicity with steatosis, as well as lactic acidosis. Treat overdose with symptomatic and supportive measures, including hemodialysis.
Description
Emtricitabine is a synthetic nucleoside inhibitor of HIV-1 reverse transcriptase. Its mechanism of action entails the phosphorylation of the oxathiolane carbinol function by cellular enzymes to form the corresponding 5′-triphosphate, which competes with the endogenous 2′-deoxycytidine 5′-triphosphate substrate. The in vitro activity of emtricitabine ranges from IC50 of 0.00013 to 0.64 mM against lymphoblastoid cell lines, MAGI-CCR5 cell lines, and peripheral blood mononuclear cells. Emtricitabine is prepared in about 16 steps from L-gulose, from which the O–C–S carbon stereochemistry is derived in the formation of substituted key 4-O-Acetyl-1,3- oxathiolane intermediate. This intermediate is coupled with N-benzoyl-O-trimethylsilyl- 5-fluoro-cytosine in the presence of trimethylsilyl triflate. The resulting anomeric mixture is separated by silica chromatography and subjected to two deprotection steps. Emtricitabine resistant isolates (M184V/I) were cross-resistant to lamivudine (desfluoro version of emtricitabine) and zalcitabine but remained sensitive to abacavir, didanosine, stavudine, tenofovir, zidovudine, and non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, and nevirapine). In a 48-week clinical trial using emtricitabine with didanosine and efavirin versus stavudine, didanosine and efavirenz produced 81 versus 68% responder rate respectively. In one study, 37.5% of treatment na?¨ve patients that were not achieving successful viral levels were found to exhibit a reduced potency toward emtricitabine. This resistance was due to M184V/I mutation in the HIV reverse transcriptase gene. Recommended dosing of emtricitabine is 200 mg (capsule) once per day. It is 93% bio-available and has a plasma half-life about 10 h, with 86% renal clearance. Side effects associated with emtricitabine treatment were generally mild to moderate and included headache, diarrhea, nausea, and rash. A generally mild and asymptomatic hyperpigmentation of the palms and/or soles was also observed.
Chemical properties
White to Off-White Crystalline Solid
Originator
Emory University (US)
The Uses of Emtricitabine
Labeled Emtricitabine, intended for use as an internal standard for the quantification of Emtricitabine by GC- or LC-mass spectrometry.
The Uses of Emtricitabine
A reverse transcriptase inhibitor. A nucleoside analog structurally related too lamivudine.It is an effective antiviral agent against HIV, HBV, and other viruses replicating in a similar manner. A nucleoside analog structurally related to Lamivudine (L172500).
The Uses of Emtricitabine
anti-Alzheimer’s treatment
Background
Emtricitabine is a nucleoside reverse transcriptase inhibitor (NRTI) indicated for the treatment of HIV infection in adults or combined with tenofovir alafenamide for the prevention of HIV-1 infection in high risk adolescents and adults. Emtricitabine is a cytidine analogue. The drug works by inhibiting HIV reverse transcriptase, preventing transcription of HIV RNA to DNA.
Emtricitabine was granted FDA approval on 2 July 2003.
Indications
Emtricitabine is indicated in combination with other medications for the treatment of HIV-1 infections; treatment of HIV-1 infections in pediatric patients 25-35kg, treatment of HIV-1 infections in adult patients ≥35kg, for pre exposure prophylaxis of HIV-1 in adolescent and adult patients excluding those who have receptive vaginal sex; treatment of HIV-1 infections in pediatric and adult patients ≥17kg, pre exposure prophylaxis in adolescents and adults ≥35kg; treatment of HIV-1 in patients ≥12 years and ≥35kg; treatment of HIV-1 in patients weighing ≥35kg; treatment of HIV-1 in patients weighing ≥25kg; and treatment of HIV-1 in patients weighing ≥40kg.
What are the applications of Application
Emtricitabine is a HIV-1 reverse transcriptase (HIV-1 RT) inhibitor that can cause termination of DNA chain elongation in viruses.
Definition
ChEBI: An organofluorine compound that is 5-fluorocytosine substituted at the 1 position by a 2-(hydroxymethyl)-1,3-oxathiolan-5-yl group (2R,5S configuration). It is used in combination therapy for the treatment of HIV-1 infect on.
brand name
Emtriva (Gilead Sciences) [Note—Emtricitabine has appeared in the literature with the trivial names, (-)-FTC and FTC-(-).].
Acquired resistance
Resistance is associated with a substitution in the HIV-1 reverse transcriptase gene at codon 184 (M184V/I). Emtricitabineresistant isolates are cross-resistant to lamivudine. HIV-1 isolates with the K65R substitution in the reverse transcriptase coding region have reduced susceptibility.
General Description
Emtricitabine is an orally active NRTI whose pharmacokineticsare favorable for once-daily administration.
Pharmaceutical Applications
A synthetic nucleoside analog of cytosine, formulated for oral use.
Pharmacokinetics
Emtricitabine is a cytidine analog that competes with the natural substrate of HIV-1 reverse transcriptase to be incorporated into newly formed DNA, terminating its transcription. It is administered once daily so it has a long duration of action. Patients should be counselled regarding the risk of lactic acidosis and hepatomegaly with steatosis.
Pharmacokinetics
Oral absorption: capsules 93%
Cmax 200 mg oral once daily: 1.8 ± 0.7 mg/L
Plasma half-life: c. 10 h
Volume of distribution: 1.4 ± 0.3 L/kg
Plasma protein binding: <4%
Absorption and distribution
It is rapidly and extensively absorbed. There is moderate CNS penetration. The estimated semen:plasma ratio is approximately 4. There are presently no data on levels in breast milk.
Metabolism and excretion
It does not inhibit human cytochrome P450 enzymes. About 80% is excreted in the urine, the rest in feces. Renal clearance is greater than the estimated creatinine clearance, suggesting elimination by both glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are renally excreted. Exposure is significantly increased in renal insufficiency, but dose reductions are not generally recommended. It is unlikely that a dose adjustment would be required in the presence of hepatic impairment.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side Effects
At least 10% of patients suffer headache, diarrhea, nausea, fatigue, dizziness, depression, insomnia, abnormal dreams, rash, abdominal pain, asthenia, increased cough and rhinitis. Skin hyperpigmentation is common (≥10%) in pediatric patients. Emtricitabine competes with lamivudine for the enzymes involved in intracellular phosphorylation; their co- administration is contraindicated.
Synthesis
Emcitritabine (VII) was discovered by researchers at Emory University and licensed to Triangle Pharmaceuticals, which started the development work before being acquired by Gilead. Because emcitritabine (VII) belongs to an important structural class of nucleosides with marketed drugs, such as 3TC, several processes for the manufacture of this class of oxathiolane nucleosides have appeared in patents and scientific literature. However, only the synthesis described in the latest patent filed for the manufacture of emcitritabine (VII) and one other efficient synthesis from the Liotta group will be described . The synthesis started with diacylation with butyryl chloride (62) of the 2-butene-1,4-diol (61) in methyl t-butylether at 0??C to room temperature in the presence of triethylamine to give diacylated product 63 in 95% yield. Ozonolysis followed by reduction with thiourea provided a mixture of hemiacetal 64 mixed with acetals, dimers and trimers in 97% yield, which was used in the next step directly. The hemiacetal mixture was reacted with thioacetic acid in toluene at 85??C for 3 hr to give the crude keto oxathiolane mixture, which was purified by vacuum distillation in a 2-in Pope Scientific wiped film still to remove impurities and collect about 92% pure 66 in 54% yield. Also mentioned in the patent is the potential use of enzymatic resolution of the isomers as reported previously. This keto oxathiolane 66 was reduced at 5??C with lithium aluminum t-butoxide, which was prepared in situ via reaction of LAH and t-butanol, and the resulting lactol was trapped with acetic anhydride in the presence of DMAP in the same reaction vessel to give, after workup, 87% yield of the key intermediate acetate 67. The bis-silyl protected 5- fluorocytosine 68, prepared in situ by reacting 5- fluorocytosine (71) with HMDS, was reacted with acetate 67 in the presence of trimethylsilyliodide at 0??C to room temperature to give a 1:1 mixture of alpha and beta-anomers 69 and 70. Pure 69 could be isolated by recrystallization from toluene. Cleavage of the butyryl group with a strongly basic DOWEX SBR resin in methanol at room temperature gave emcitritabine (VII) in 81% yield. An alternate concise synthesis reported by Liotta et al is worth mentioning. This synthetic route accessed the key thioxalane acetate 76 as the TBDMS ether in four steps from allyl alcohol 72. The key step to the preparation of the final compound was the coupling of the bis-silyl 5-fluorocytosine (68) with acetate 76 with tin tetrachloride in a stereoselective manner, after cleavage of the silyl groups and recrystallization, to give pure cis isomer emcitritabine (VII) in excellent yield.
Drug interactions
Potentially hazardous interactions with other drugs Antivirals: avoid concomitant use with lamivudine. Orlistat: absorption of emtricitabine possibly reduced.
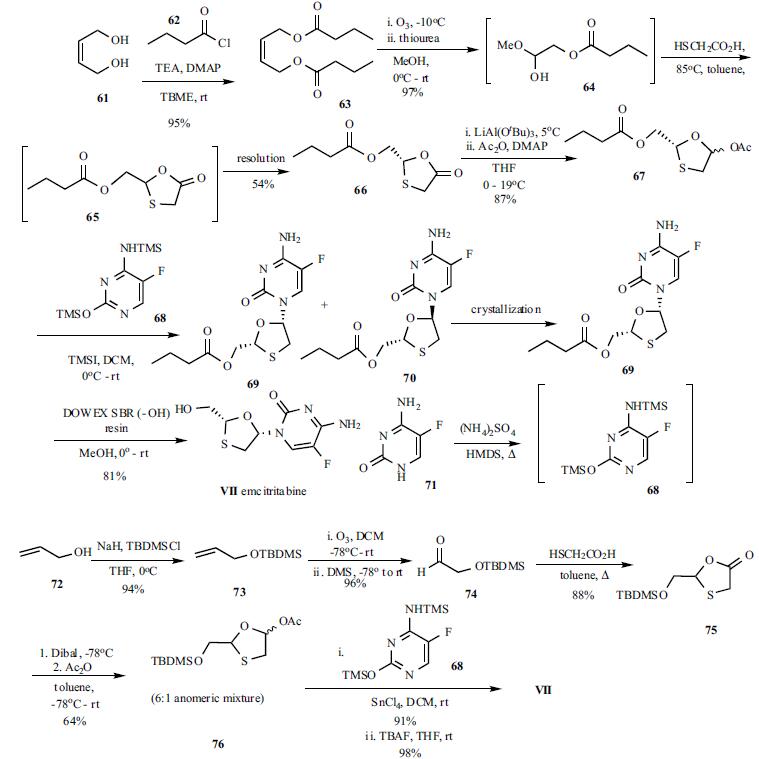
Metabolism
Emtricitabine is approximately 86% unmetabolized. Approximately 9% of a dose is metabolized to 3'-sulfoxide diastereomers, 4% to the 2'-O-glucuronide, and a minor amount is converted to 5-fluorocytosine.
Metabolism
There is limited metabolism of emtricitabine. The biotransformation of emtricitabine includes oxidation of the thiol moiety to form the 3'-sulphoxide diastereomers (approximately 9% of dose) and conjugation with glucuronic acid to form 2'-O-glucuronide (approximately 4% of dose). Emtricitabine is primarily excreted by the kidneys with complete recovery of the dose achieved in urine (approximately 86%) and faeces (approximately 14%). 13% of the emtricitabine dose was recovered in urine as three metabolites
Properties of Emtricitabine
| Melting point: | 136-140°C |
| Boiling point: | 443.3±55.0 °C(Predicted) |
| alpha | D25 -133.60° (c = 0.23 in MeOH) |
| Density | 1.82±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly) |
| form | Solid |
| pka | 13.83±0.10(Predicted) |
| color | White to Pale Yellow |
| λmax | 280nm(H2O)(lit.) |
| Merck | 14,3565 |
| CAS DataBase Reference | 143491-57-0(CAS DataBase Reference) |
Safety information for Emtricitabine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Emtricitabine
| InChIKey | XQSPYNMVSIKCOC-NTSWFWBYSA-N |
Emtricitabine manufacturer
Saimak Laboratories
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(2S)-2-[[[(1,1-Dimethylethyl)diphenylsilyl]oxy]methyl]-1,3-oxathiolan-5-ol 5-Acetate](https://img.chemicalbook.in/CAS/20180601/GIF/202532-88-5.gif)
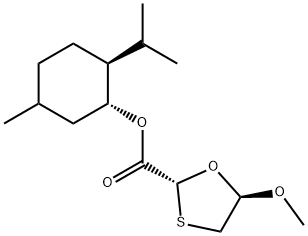
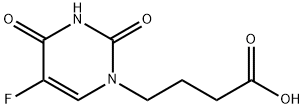
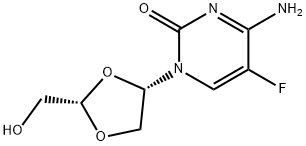
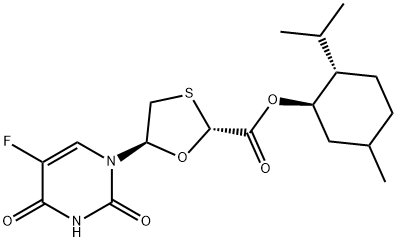
You may like
-
 Emtricitabine 98%View Details
Emtricitabine 98%View Details
143491-57-0 -
 Emtricitabine 98%View Details
Emtricitabine 98%View Details -
 Emtricitabine 98%View Details
Emtricitabine 98%View Details
143491-57-0 -
 143491-57-0 EMTRICITABINE 95-99%View Details
143491-57-0 EMTRICITABINE 95-99%View Details
143491-57-0 -
 Emtricitabine CAS 143491-57-0View Details
Emtricitabine CAS 143491-57-0View Details
143491-57-0 -
 Emtricitabine 98% CAS 143491-57-0View Details
Emtricitabine 98% CAS 143491-57-0View Details
143491-57-0 -
 Emtricitabine CAS 143491-57-0View Details
Emtricitabine CAS 143491-57-0View Details
143491-57-0 -
 4-Amino-5-Fluoro-1-(2-(Hydroxymethyl)-1,3-Oxathiolan-5-yl)-(2RCis)-2(1H)-Pyrimidinone in hyderabadView Details
4-Amino-5-Fluoro-1-(2-(Hydroxymethyl)-1,3-Oxathiolan-5-yl)-(2RCis)-2(1H)-Pyrimidinone in hyderabadView Details
143491-57-0
