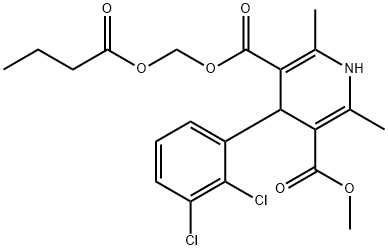
Clevidipine butyrate
- CAS NO.:167221-71-8
- Empirical Formula: C21H23Cl2NO6
- Molecular Weight: 456.32
- MDL number: MFCD00940070
- EINECS: 682-702-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
What is Clevidipine butyrate?
Description
Clevidipine is an ultra-short-acting vasodilator of the dihydropyridine
(DHP) class of CCB. It is indicated as an intravenous treatment for the
acute management of hypertension when oral therapy is not feasible or
not desirable. Clevidipine is the second intravenous CCB to be marketed
behind nicardipine for this indication. Both agents are primarily used
for urgent treatment of hypertension in cardiac surgical setting, intensive
care units, and emergency departments. Other intravenous agents
currently on the market for this indication include sodium nitroprusside,
nitroglycerin, fenoldopam, hydralazine, esmolol, and labetalol. Clevidipine is a potent, arterial-specific, vasodilator with very little or no effect
on the myocardial contractility and venous capacitance. It has a
rapid onset and offset of action and a very short plasma half-life, which
allows for titration of the drug to achieve precise BP control.
clevidipine is exclusively metabolized in the blood and the tissues. It does not undergo any renal or hepatic metabolism and does not accumulate in the body. Clevidipine is a blocker of L-type calcium channels, which mediate the influx of calcium during depolarization in arterial smooth muscle.
Chemical properties
White to Off-White Solid
Originator
AstraZeneca (United Kingdom)
The Uses of Clevidipine butyrate
Clevidipine butyrate is a dihydropyridine calcium channel blocker use as agent for the reduction of blood pressure。
The Uses of Clevidipine butyrate
Calcium channel blocker, used as an oral antihypertensive.
The Uses of Clevidipine butyrate
Clevidipine butyrate is a novel intravenous, short-acting calcium channel blocker that provides quick and accurate control of blood pressure in an emergency setting. Unlike other intravenous hypertension drugs which are metabolized in kidney or liver and tend to accumulate in the body, clevidipine is metabolized in the blood and tissues and thus avoids accumulation. The drug was launched by Medicines Company which aquired the rights from AstraZeneca.
The Uses of Clevidipine butyrate
A calcium channel protein inhibitor and blocker
Background
Clevidipine is a dihydropyridine L-type calcium channel blocker that is selective for vascular smooth muscle and is indicated for blood pressure reduction when oral therapy is not an option.
Indications
For the reduction of blood pressure when when oral antihypertensive therapy is not feasible or not desirable.
What are the applications of Application
Cleviprex is a calcium channel protein inhibitor and blocker
Definition
ChEBI: Clevidipine is a dihydropyridine.
brand name
Clevelox (The Medicines Company).
Pharmacokinetics
Clevidipine belongs to a well-known class of drugs called dihydropyridine calcium channel antagonists. Clevidpine is the first third generation intravenous dihydropyridine calcium channel blocker. In vitro studies demonstrated that clevidipine acts by selectively relaxing the smooth muscle cells that line small arteries, resulting in arterial dilation, widening of the artery opening, and without reducing central venous pressure or reducing cardiac output.
Side Effects
Clevidipine is contraindicated in patients with severe aortic stenosis, defective lipid metabolism, and allergies to eggs, egg products, soybeans, or soy products. The chemical synthesis of clevidipine entails the esterification of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid monomethyl ester with chloromethyl butyrate by the action of potassium bicarbonate in refluxing acetonitrile.
Synthesis
The chemical synthesis of clevidipine entails the esterification of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid monomethyl ester with chloromethyl butyrate by the action of potassium bicarbonate in refluxing acetonitrile. The DHP-monoester starting material is obtained in two steps through condensation of methyl 2,3-dichlorobenzylidine acetoacetate and 2-cyanoethyl ester of 2-amino-2-propenoic acid to the DHP diester, followed by base catalyzed cleavage of the cyanoethyl group. Clevidipine is practically insoluble in water and is formulated in an oil-inwater emulsion.
Metabolism
Clevidipine is rapidly hydrolyzed to inactive metabolites by esterases in arterial blood.
Properties of Clevidipine butyrate
| Melting point: | 128-130°C |
| Boiling point: | 539.7±50.0 °C(Predicted) |
| Density | 1.289 |
| Flash point: | 280℃ |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| Water Solubility | Insoluble in water |
| solubility | ≥21.4 mg/mL in DMSO; insoluble in H2O; ≥7.45 mg/mL in EtOH with ultrasonic |
| pka | 2.45±0.70(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
| CAS DataBase Reference | 167221-71-8 |
Safety information for Clevidipine butyrate
Computed Descriptors for Clevidipine butyrate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Clevidipine butyrate 99% (HPLC) CAS 167221-71-8View Details
Clevidipine butyrate 99% (HPLC) CAS 167221-71-8View Details
167221-71-8 -
 Clevidipine CAS 167221-71-8View Details
Clevidipine CAS 167221-71-8View Details
167221-71-8 -
 Clevidipine Butyrate CAS 167221-71-8View Details
Clevidipine Butyrate CAS 167221-71-8View Details
167221-71-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
