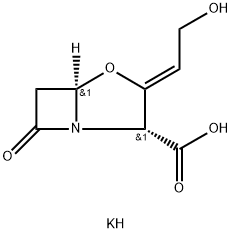
Clavulanic acid
- CAS NO.:58001-44-8
- Empirical Formula: C8H9NO5
- Molecular Weight: 199.16
- MDL number: MFCD00867004
- EINECS: 261-069-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
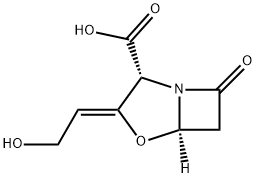
What is Clavulanic acid?
Absorption
Clavulanic acid, when taken orally, is well absorbed in the gastrointestinal tract. After administration of radiolabeled clavulanic acid to four human subjects, a minimum of 73% absorption and the average absolute bioavailability was calculated at 64%. The mean Cmax in a group of 8 healthy research volunteers was 2.098 ± 0.441 micrograms/ml in a pharmacokinetic study. The same study reported a mean Tmax of 1.042 ± 0.80 hours. Tmax is reported to be 40-120 minutes according to another pharmacokinetic study.
Toxicity
LD50 information
Clavulanic acid has demonstrated low oral acute toxicity in adult rodents, having an LD50 of more than 2000 mg/kg. The toxicity of clavulanic acid on pre-weaning rats was also studied. Gastrointestinal disturbance and mortality occurred, even at lower clavulanic acid doses of 125 mg/kg.
Overdose information
Overdose information has been obtained for the combination of amoxicillin and clavulanic acid, as these drugs are frequently administered together in a single product. Changes in fluid and electrolyte balances and gastrointestinal symptoms may occur in the case of an overdose. Offer symptomatic treatment or gastrointestinal disturbances, while considering the importance of fluid and electrolyte balance. This drug may be removed by a session of hemodialysis. When coadministered with amoxicillin, crystalluria causing renal failure has been observed. Seizures may also occur in a case of overdose, or in a patient with renal failure.
Description
Clavulanic acid is a mold product with only weak intrinsic antibacterial activity, but it is an excellent irreversible inhibitor of most β-lactamases. It is believed to acylate the active site serine by mimicking the normal substrate. Hydrolysis occurs with some β-lactamases, but in many cases, subsequent reactions occur that inhibit the enzyme irreversibly. This leads to its classification as a mechanism-based inhibitor (or so-called suicide substrate). The precise chemistry is not well understood, but when clavulanic acid is added to ampicillin and amoxicillin preparations, the potency against β-lactamase–producing strains is markedly enhanced.
Originator
Augmentin,Beecham,UK,1981
The Uses of Clavulanic acid
beta-lactamase inhibitor
Indications
Clavulanic acid combined with other antibiotics is indicated to prevent the development of drug-resistant strains of bacteria and promotes their therapeutic antibacterial effects.
The following conditions, when they produced beta-lactamases, have been treated with a combination of amoxicillin and clavulanic acid or ticarcillin and clavulanic acid:
Acute otitis media caused by H. influenzae and M. catarrhalis
Sinusitis due to H. influenzae and M. catarrhalis
Lower respiratory tract infections due to Haemophilus influenzae, S.aureus, Klebsiella species, and Moraxella catarrhalis
Skin and skin structure infections caused by Staphylococcus aureus, Escherichia coli, and Klebsiella species
Urinary Tract Infections due to E. coli, Klebsiella species of bacteria, and Enterobacter species of bacteria, S.marcescens, or S.aureus
Gynecologic infections due to a variety of bacteria, including P.melaninogenicus, Enterobacter species, E.Coli species, Klebsiella species, S. aureus, S.epidermidis
Septicemia due to a variety of bacteria, including Klebsiella species, E.Coli species, S.aureus, or Pseudomonas species
Bone and joint infections due to S.aureus
Intraabdominal infections due to E.Coli, K.pnemoniae, or B.fragilis group
A note on susceptibility
It should be noted that it is only to be administered in infections that are confirmed or highly likely to be caused by susceptible bacteria. Culture and susceptibility tests should be performed if possible and used in selecting whether this antibiotic is prescribed. When beta-lactamase enzyme production is not detected during microbiological testing, clavulanic acid should not be used. When these tests are not available patterns of local infection and susceptibility may be used to determine the appropriateness of using clavulanic acid. Ticarcillin with clavulanate has shown particular efficacy in mixed infections in addition to empiric therapy before determining the susceptibility of causative organisms. The ticarcillin-clavulanic acid combination may prove to be an effective single-agent antibiotic therapy to treat infections where a regimen of several drugs may normally be used.
Background
Clavulanic acid is a beta-lactamase inhibitor that is frequently combined with Amoxicillin or Ticarcillin to fight antibiotic resistance by preventing their degradation by beta-lactamase enzymes, broadening their spectrum of susceptible bacterial infections. Clavulanic acid is derived from the organism Streptomyces clavuligerus.When it is combined with amoxicillin, clavulanic acid is frequently known as Augmentin, Co-Amoxiclav, or Clavulin.
Definition
ChEBI: Antibiotic isolated from Streptomyces clavuligerus. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes.
Manufacturing Process
100 ml of sterile water was added to a sporing culture which had been grown
on Bennetts agar in a Roux bottle for 10 days at 26°C. A mycelium/spore
suspension was produced and used to inoculate 75 liters of steam sterilized
medium of the following composition in tap water.
Arkasoy is soybean flour supplied by British Arkady Co., Old Trafford,
Manchester, UK
The pH of the medium was adjusted to 7.0
The medium was contained in a 100 liter stainless steel baffled fermenter,
agitated by a 7.5 inch vaned disc impeller at 140 rpm. Sterile air was supplied
at 75 liters per minute and the tank incubated for 72 hours at 26°C.
The contents of the seed fermenter were used to inoculate 1,500 liters of
steam sterilized medium of the following composition in tap water.
10% Pluronic L81 in soybean oil 0.2% V/V
The pH of the medium was adjusted to 7.0
The medium was contained in a 2,000 liter stainless steel fully baffled
fermenter agitated by two 19 inch vaned disc impellers at 106 rpm.
Sterile air was supplied at 1,200 liters per minute. Antifoam was added in 25
ml amounts as required. (10% Pluronic L81 in soybean oil). The fermentation
was controlled at 26°C until a maximum yield of clavulanic acid was obtained
between 3-5 days when 200-300 μg/ml of clavulanic acid were produced.
Therapeutic Function
Antibacterial
World Health Organization (WHO)
The amoxicillin/clavulanic acid combination should be reserved for infections likely or known to be caused by amoxicillin- resistant beta-lactamase producing strains. Amoxicillin/clavulanic acid is listed in the WHO Model List of Essential Drugs.
Antimicrobial activity
It exhibits broad-spectrum but low intrinsic activity, most MICs being in the range 16–128 mg/L. Enterobacteriaceae and Staph. aureus are among the more sensitive and Ps. aeruginosa the most resistant organisms. MICs of 8 mg/L against H. influenzae and 0.1–4 mg/L for penicillinase-producing N. gonorrhoeae are notable.
General Description
Clavulanic acid was found in the culture broth of Streptomyces clavuligerus by Beecham Research Laboratories in 1976. It was the first β-lactamase inhibitor. This antibiotic shows weak antibacterial activity against grampositive and gram-negative organisms but strong inhibitory activity against the β-lactamase produced by ampicillin-resistant bacteria. Clavulanic acid is used orally in combination with amoxicillin and with ticarcillin by injection to enhance the activities of these antibiotics against resistant infections.
Pharmacokinetics
Clavulanic acid inactivates some beta-lactamase enzymes that are produced by bacteria, therefore preventing enzymatic destruction of amoxicillin. This helps to treat a variety of bacterial infections which would otherwise be resistant to antibiotics without the addition of clavulanic acid.
Pharmacokinetics
It exhibits broad-spectrum but low intrinsic activity, most MICs being in the range 16–128 mg/L. Enterobacteriaceae and Staph. aureus are among the more sensitive and Ps. aeruginosa the most resistant organisms. MICs of 8 mg/L against H. influenzae and 0.1–4 mg/L for penicillinase-producing N. gonorrhoeae are notable.
Clinical Use
Clavulanic acid is an antibiotic isolated from Streptomycesclavuligeris. Structurally, it is a 1-oxopenam lacking the6-acylamino side chain of penicillins but possessing a 2-hydroxyethylidene moiety at C-2. Clavulanic acid exhibitsvery weak antibacterial activity, comparable with that of6-APA and, therefore, is not useful as an antibiotic. Itis, however, a potent inhibitor of S. aureus β-lactamaseand plasmid-mediated β-lactamases elaborated by Gramnegativebacilli.
Combinations of amoxicillin and the potassium saltof clavulanic acid are available (Augmentin) in variousfixed-dose oral dosage forms intended for the treatment ofskin, respiratory, ear, and urinary tract infections causedby -lactamase–producing bacterial strains. These combinationsare effective against β-lactamase–producingstrains of S. aureus, E. coli, K. pneumoniae, Enterobacter,H. influenzae, Moraxella catarrhalis, and Haemophilusducreyi, which are resistant to amoxicillin alone. The oral bioavailability of amoxicillin and potassium clavulanate issimilar. Clavulanic acid is acid-stable. It cannot undergo penicillanicacid formation because it lacks an amide side chain.Potassium clavulanate and the extended-spectrum penicillinticarcillin have been combined in a fixed-dose, injectableform for the control of serious infections caused byβ-lactamase–producing bacterial strains. This combinationhas been recommended for septicemia, lower respiratory tractinfections, and urinary tract infections caused by β-lactamase–producing Klebsiella spp., E. coli, P. aeruginosa,and other Pseudomonas spp., Citrobacter spp., Enterobacterspp., S. marcescens, and S. aureus. It also is used in bone andjoint infections caused by these organisms. The combinationcontains 3 g of ticarcillin disodium and 100 mg of potassiumclavulanate in a sterile powder for injection (Timentin).
Synthesis
Clavulanic acid is isolated from Streptomyces clavuligerus [60–66], and sulbactam, a sulfone of penicillanic acid, is synthesized from 6-APA [67–69]. Both compounds have extremely weak antibacterial properties and act by forming irreversible complexes with beta-lactamase, which inactivates the enzyme, and as a result the beta-lactam antibiotic has time to destroy the microorganism.
Metabolism
Clavulanic acid is heavily metabolized to form the metabolites 2,5-dihydro-4-(2- hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one. The first metabolite was found to account for 15.6% of the dose while the second metabolite was reported to account for 8.8% of the dose in one pharmacokinetic study.
Properties of Clavulanic acid
| Boiling point: | 545.8±50.0 °C(Predicted) |
| Density | 1.65±0.1 g/cm3(Predicted) |
| storage temp. | 4°C, protect from light, stored under nitrogen |
| solubility | H2O : 50 mg/mL (251.05 mM; Need ultrasonic) |
| pka | 3.68±0.20(Predicted) |
| form | Solid |
| color | Off-white to light yellow |
Safety information for Clavulanic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Clavulanic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
