Potassium clavulanate
Synonym(s):Clavulanate potassium
- CAS NO.:61177-45-5
- Empirical Formula: C8H10KNO5
- Molecular Weight: 239.27
- MDL number: MFCD01710901
- EINECS: 262-640-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-07 19:25:08
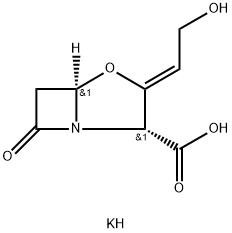
What is Potassium clavulanate?
Description
Clavulanate is a β-lactamase inhibitor that is effective against Ambler class A β-lactamases (IC50 values range from 12 to 60 nM). While it is not an effective antibiotic by itself, clavulanate is commonly used with other antibiotics that would be inactivated by β-lactamases secreted by bacteria. It is often combined with amoxicillin and other penicillin-based antibiotics.
Chemical properties
Pale Yellowish Solid
Originator
Clavulanate ,potassium Lek
The Uses of Potassium clavulanate
Clavulanic Acid is a β-lactamase inhibitor, typically added to amoxicillin to increase its effectiveness.
The Uses of Potassium clavulanate
Clavulanic acid is a β-lactam antibiotic produced by several species of Streptomyces. The free acid degrades and is isolated and maintained as either the sodium or potassium salt. Clavulanate is a weak antibiotic, but is a potent inhibitor of β-lactamases. In combination with penicillin and cephalosporins, it shows potent synergistic activity. Clavulanic acid is a suicide inhibitor, covalently binding to a serine residue in the active site of the β-lactamase.
The Uses of Potassium clavulanate
A -Lactamase inhibitor, typically added to amoxicillin to increase its effectiveness
Definition
ChEBI: A potassium salt having clavulanate as the counterion. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes and has only weak anitbiotic activity when administered alone. However it can be used in combination with amoxicillin trihydrate (under the trade name Augmentin) for treatment of a variety of bacterial infections, where it prevents antibiotic inactivation by microbial lactamases.
What are the applications of Application
Clavulanic acid potassium salt is a β-Lactamase inhibitor effective against Ambler class A β-lactamases.
Manufacturing Process
Clavulanic acid may be obtained by aerobic cultivation of Streptomyces
clavuligerus in conventional nutrient media at, for example, about 25°-30°C
under roughly neutral conditions.
Cultivation of Streptomyces clavuligeru:
Streptomyces clavuligerus was cultivated at 26°C on agar slopes containing
1% Yeatex (yeast extract) ("Yeatex" is a Registered Trade mark), 1% glucose
and 2% Oxoid agar No. 3, pH 6.8. A sterile loop was used to transfer
mycelium and spores from the slope into 100 ml of a liquid medium in a 500
ml Ehrlenmeyer flask. The liquid medium had the following composition: Oxoid
Malt Extract 10g/L, Oxoid Bacteriological Peptone 10g/L, Glycerol 20 g/L, Tap
water 1 L.
The medium was adjusted to pH 7.0 with sodium hydroxide solution and 100
ml volumes dispensed into flasks which were closed with foam plugs prior to
autoclaving at 15 lb/sq.in. for 20 min. An inoculated seed flask was shaken for
3 days at 26°C on a rotary shaker with a 2 inch throw and a speed of 240
r.p.m.
Production stage flasks containing the liquid medium described above were
inoculated with 5% vegetative inoculum and grown under the same conditions
as the seed flask.
Clavulanic acid may be extracted from the culture medium. Normally the cells
of the Streptomyces clavuligerus are first removed from culture medium by
filtration or centrifugation. Then clavulanic acid is extracted into an organic
solvent, for example, n-butanol or ethyl acetate, or n-butyl acetate, or methyl
isobutyl ketone. Then n-butanol fraction are treated with new aqueous phase
using potassium hydrogen carbonate and then this aqueous phase is washed
with n-butanol. This aqueous extract, after separation of the phases, is
concentrated under reduced pressure. Freeze-drying at -20°C may also be
employed to provide a solid crude preparation of the potassium Z-(2R,5R)-3-
(β-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3,2,0]heptane-2-carboxylate
(clavulanate potassium).
Therapeutic Function
Beta-lactamase inhibitor
Properties of Potassium clavulanate
| Melting point: | >1600C (dec) |
| alpha | +55~+60° |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Freely soluble in water, slightly soluble in ethanol (96 per cent), very slightly soluble in acetone. |
| form | neat |
| form | Solid |
| color | Light brown to brown |
| CAS DataBase Reference | 61177-45-5(CAS DataBase Reference) |
Safety information for Potassium clavulanate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids H252:Self-heating substances and mixtures H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Potassium clavulanate
| InChIKey | ABVRVIZBZKUTMK-JSYANWSFSA-M |
Potassium clavulanate manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 61177-45-5 Clavulanate Potassium 99%View Details
61177-45-5 Clavulanate Potassium 99%View Details
61177-45-5 -
 Potassium clavulanate 96% CAS 61177-45-5View Details
Potassium clavulanate 96% CAS 61177-45-5View Details
61177-45-5 -
 Clavulanate potassium CAS 61177-45-5View Details
Clavulanate potassium CAS 61177-45-5View Details
61177-45-5 -
 Potassium clavulanate CAS 61177-45-5View Details
Potassium clavulanate CAS 61177-45-5View Details
61177-45-5 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
