Voglibose
Synonym(s):3,4-Dideoxy-4-{[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-2-D -epi-inositol;N-(1,3-Dihydroxyprop-2-yl)valiolamine
- CAS NO.:83480-29-9
- Empirical Formula: C10H21NO7
- Molecular Weight: 267.28
- MDL number: MFCD00865496
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
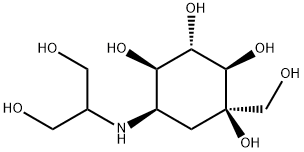
What is Voglibose?
Description
Voglibose was introduced in Japan for the treatment of postprandial hyperglycemia in diabetic patients. As an orally active α-D-glucosidase inhibitor, it is effective in decreasing the release of glucose from carbohydrate ingestion and slowing the increase of postprandial blood glucose levels, as demonstrated in animals and humans following sucrose and starch loading. In addlion, voglibose was reported to have prophylactic effect in genetically obese Wistar rats, maintaining low plasma levels of glucose, triglyceride and insulin, indicating a potential usefulness for the management of other carbohydrate-dependent metabolic disorders such as obesity. Voglibose is much more potent and has fewer side effects than acarbose, another agent in its class.
Chemical properties
Colourless Crystalline Solid
Originator
Takeda (Japan)
The Uses of Voglibose
An alpha-Glucosidase inhibitor used as an antidiabetic
The Uses of Voglibose
Voglibose is an α-glucosidase inhibitor, similar to acarbose and miglitol, used for lowering post-prandial hyperglycemia (PPHG) in people with diabetes mellitus. Voglibose is used to study it benefits as a protectant against ischemia-reperfusion injury through glucagon-like peptide 1 receptors and the phosphoinositide 3-kinase-Akt-endothelial nitric oxide synthase pathway.
The Uses of Voglibose
An α-Glucosidase inhibitor used as an antidiabetic.
The Uses of Voglibose
For the treatment of diabetes. It is specifically used for lowering post-prandial blood glucose levels thereby reducing the risk of macrovascular complications.
What are the applications of Application
Voglibose is an α-glucosidase inhibitor that increases glucagon-like peptide 1 secretion
Definition
ChEBI: Voglibose is an organic molecular entity.
Manufacturing Process
N-(1,3-Dihydroxy-2-propyl)valiolamine:
To a solution of 2.0 g of valiol amine in 50 ml of N,N-dimethylformamide are
added 3.4 g of dihydroxyacetone, 1.5 ml of 2 N hydrochloric acid and 2.6 g of
sodium cyanoborohydride, followed by stirring at 60 to 70°C for 16 hours. The
reaction solution is concentrated under reduced pressure to distill off the N,N-
dimethylformamide as much as possible, and the residue is dissolved in 100 ml of water. The solution is made acid with 2 N hydrochloric acid, stirred for
30 to 40 minutes under ice-cooling, adjusted to pH 4.5 with 1 N sodium
hydroxide solution and subjected to column chromatography (250 ml) on
Dowex 50W x 8 (H + type) (produced by Dow Chemical of the United States of
America). After the washing with water, the elution is carried out with 0.5 N
aqueous ammonia. The eluate is concentrated under reduced pressure, and
the concentrate is chromatographed on a column (250 ml) of Amberlite CG-50
(NH 4 + type) (produced by Rohm and Haas Co. of the United States of
America), followed by the elution with water. The eluate is concentrated under
reduced pressure, and the concentrate is lyophilized to give 2.0 g of white
powder of N-(1,3-dihydroxy-2-propyl)valiol amine.
Ethanol (about 60 ml) is added to the above lyophilized product (1.2 g) of N-
(1,3-dihydroxy-2-propyl)valiol amine, and the mixture is warmed for 30
minutes in a hot water bath (the bath temperature: 90-95°C), followed by
leaving on standing in a refiegerator. The resultant crystalline substance is
recovered by filtration, washed with ethanol and then dried in a desiccator
under reduced pressure. Yield of 0.95 g. MP: 162-163°C. [α] D 25 = +26.2° (c
= 1, H 2 O).
brand name
Basen (Takeda); Glustat (Takeda).
Therapeutic Function
Antidiabetic, Antiobesity
World Health Organization (WHO)
Acarbose and voglibose area-glucosidase inhibitors and delay digestion/absorption of carbohydrates as well as improving postprandial hyperglycaemia.
General Description
The most potent -glucosidase inhibitor is the valiolamine derivative voglibose. Compared with acarbose its inhibitory potency in vitro, depending on the -glucosidase enzyme, is up to 200 times higher. In Japan, daily dosages are only 0.3 – 0.9 mg, however, therapeutic efficacy on HbA1c reduction could not be clearly demonstrated with those dosages. HbA1c, a glycated hemoglobin species, which occurs in blood, is a diagnostic measure for the non-enzymatic glycation process, and depends on the plasma glucose concentration. The process of protein glycation is discussed to be responsible for the development of diabetic complications (neuropathy, retinopathy, nephropathy). In Europe voglibose is under evaluation with distinctly higher daily dosages of 1.5 to 6 mg. In contrast to acarbose, the smaller inhibitors of the monosaccharide type (miglitol, emiglitate, voglibose) do not inhibit pancreatic -amylase.
Biological Activity
Orally active α -glucosidase inhibitor (IC 50 values are 3.9 and 6.4 nM at sucrase and maltase respectively). Increases glucagon-like peptide 1 (GLP-1) secretion and decreases food consumption in ob/ob mice, and reduces plasma concentrations of glucose, triglycerides and insulin in Wistar fatty rats. Exhibits antidiabetic and antiobesity activity in vivo .
Properties of Voglibose
| Melting point: | 162-163°C |
| alpha | D25 +26.2° (c = 1 in water) |
| Boiling point: | 601.9±55.0 °C(Predicted) |
| Density | 1.58±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Heated), Water (Sparingly, Sonicated) |
| form | Solid |
| pka | 13.26±0.70(Predicted) |
| color | White to Off-White |
| Merck | 14,10029 |
| CAS DataBase Reference | 83480-29-9(CAS DataBase Reference) |
Safety information for Voglibose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Voglibose
| InChIKey | FZNCGRZWXLXZSZ-CIQUZCHMSA-N |
Voglibose manufacturer
Ralington Pharma
Beloorbayir Biotech Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![D-epi-Inositol, 1-O-(cyclohexylmethyl)-3,4-dideoxy-4-[[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-2-C-(hydroxymethyl)- (9CI)](https://img.chemicalbook.in/CAS/20200611/GIF/756527-25-0.gif)
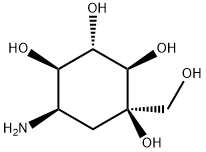
![D-epi-Inositol, 2-C-[(cyclohexylmethoxy)methyl]-3,4-dideoxy-4-[[2-hydroxy-1-(hydroxymethyl)ethyl]amino]- (9CI)](https://img.chemicalbook.in/CAS/20200611/GIF/679391-81-2.gif)
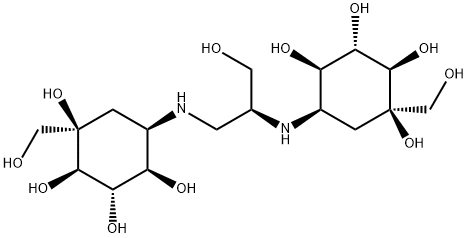
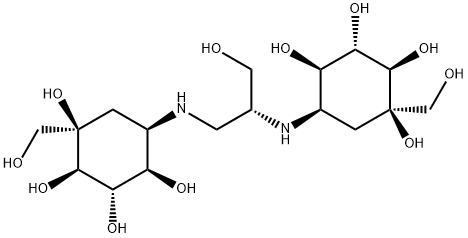
You may like
-
 83480-29-9 Voglibose 98%View Details
83480-29-9 Voglibose 98%View Details
83480-29-9 -
 Voglibose 99%View Details
Voglibose 99%View Details -
 Voglibose 98%View Details
Voglibose 98%View Details
83480-29-9 / 112653-29-9 -
 83480-29-9 / 112653-29-9 Voglibose 98%View Details
83480-29-9 / 112653-29-9 Voglibose 98%View Details
83480-29-9 / 112653-29-9 -
 83480-29-9 / 112653-29-9 98%View Details
83480-29-9 / 112653-29-9 98%View Details
83480-29-9 / 112653-29-9 -
 Voglibose 98%View Details
Voglibose 98%View Details
83480-29-9 / 112653-29-9 -
 Voglibose extrapure CAS 83480-29-9View Details
Voglibose extrapure CAS 83480-29-9View Details
83480-29-9 -
 Voglibose CAS 83480-29-9View Details
Voglibose CAS 83480-29-9View Details
83480-29-9