Carboprost tromethamine
Synonym(s):(15S)-15-methyl prostaglandin F2α tromethamine;(5Z,9α,11α,13E,15S)-9,11,15-Trihydroxy-15- methylprosta-5,13-dien-1-oic acid with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1);(5Z,9a,11a,15E,15S)-9,11,15-Trihydroxy-15-methylprosta-5,13-dien-1-oic acid tris(hydroxymethyl)aminomethane salt;Carboprost trometamol;Carboprost tromethamine
- CAS NO.:58551-69-2
- Empirical Formula: C25H47NO8
- Molecular Weight: 489.65
- MDL number: MFCD01694769
- EINECS: 200-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
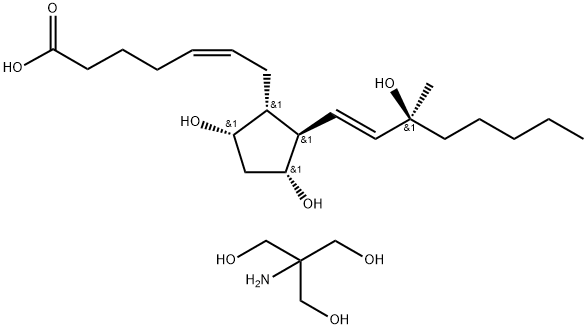
What is Carboprost tromethamine?
Toxicity
Symptoms of overdose include irritation, nausea, vomiting, diarrhea, coughing, dyspnea, asthma, hypertension, flushing, and pyrexia.
Chemical properties
White or almost white powder.
Originator
Hemabate,Pharmacia and Upjohn Company
The Uses of Carboprost tromethamine
Oxytocic.
The Uses of Carboprost tromethamine
Carboprost Tromethanimine is the salt of Carboprost (C177580), an analog of prostaglandin F2α.
Background
A nonsteroidal abortifacient agent that is effective in both the first and second trimesters of pregnancy.
Indications
For aborting pregnancy between the 13th and 20th weeks of gestation as calculated from the first day of the last normal menstrual period and in the following conditions related to second trimester abortion: 1. Failure of expulsion of the fetus during the course of treatment by another method; 2. Premature rupture of membranes in intrauterine methods with loss of drug and insufficient or absent uterine activity; 3. Requirement of a repeat intrauterine instillation of drug for expulsion of the fetus; 4. Inadvertent or spontaneous rupture of membranes in the presence of a previable fetus and absence of adequate activity for expulsion. Also for the treatment of postpartum hemorrhage due to uterine atony which has not responded to conventional methods of management.
Definition
ChEBI: The tromethamine salt of carboprost. It is used as an abortifacient agent that is effective in both the first and second trimesters of pregnancy.
Manufacturing Process
Carboprost tromethamine was prepared by mixing of (15S)-15-methyl-PGF2α methyl esthers with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1).
(15R)- and (15S)-15-methyl-PGF2α methyl esthers:
A mixture of 0.23 g sodium hydride (50% dispersion in mineral oil) and 10 ml of dimethyl sulfoxide stirred under nitrogene at 70-75°C. After 1 hours, gas evolution had ceased. After 0.5 hour, the mixture was cooled to room temperature. To the mixture was added 1.06 g 4- carboxybutyltriphenylphosphonium bromide and the resulting dark red solution stirred 0.5 hour. To this solution was added a solution of 0.48 g of (-)-3α,5α-dihydroxy-2β-[3-(RS)-3-hydroxy-3-methyl-trans-octenyl] cyclopentane-1α-acetaldehyde γ-lactol 3-benzoate in 15 ml of dimethyl sulfoxide. The resulting dark orange mixture was stirred at room temperature for 12 hours. Another 1.2 mmol of freshly prepared ilide in 3.6 ml of solution (prepared as above) was added. After 24 hours, the reaction was quenched by addition to 15 ml of 2 M sodium bisulfate (diluted with ice water) and 25 ml of ether. The organic extract was washed with 5 ml of 1 N sodium hydroxide, and twice with water. The aqueous washings were combined (pH £ 11) and acidified with sodium bisulfate to pH about 1 in the presence of ether. The aqueous phase was extracted with ether. The extract was evaporated to give 0.45 g of dark oily solid. The crude product was dissolved in a mixture of methylene chloride, ether and methanol and treated with excess etheral diazomethane. Evaporation gave 0.40 g of dark oil. The crude product was chromatographed on 10 g of silica gel. Fraction 9-12 contained a mixture of epimers (15R)- and (15S)-15-methyl-PGF2α-methyl esthers, yield 160 mg (35%) as an oil. The structure of product was confirmed by 1H-NMR spectrum.
brand name
Hemabate (Pharmacia & Upjohn).
Therapeutic Function
Oxytocic
General Description
Carboprost tromethamine,15-(S)-methyl-PGF2α (Hemabate), is a prostaglandin derivativethat has been modified to prevent metabolic oxidation ofthe 15-position alcohol function. This derivative may be administeredin a hospital setting only in a dose of 250 μg bydeep intramuscular injection to induce abortion or to amelioratesevere postpartum hemorrhage.
Pharmacokinetics
Carboprost tromethamine administered intramuscularly stimulates in the gravid uterus myometrial contractions similar to labor contractions at the end of a full term pregnancy. Whether or not these contractions result from a direct effect of carboprost on the myome-trium has not been determined. Nonetheless, they evacuate the products of conception from the uterus in most cases. Postpartum, the resultant myometrial contractions provide hemostasis at the site of placentation. It also stimulates the smooth muscle of the human gastrointestinal tract. This activity may produce the vomiting or diarrhea or both that is common when carbo-prost tromethamine is used to terminate pregnancy and for use postpartum. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost trometh-amine used for the termination of pregnancy, and for use postpartum, some patients do experience transient temperature increases. In laboratory animals and in humans large doses of carboprost tromethamine can raise blood pressure, probably by contracting the vascular smooth muscle. With the doses of carboprost tromethamine used for terminating pregnancy, this effect has not been clinically significant. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost tromethamine used for the termination of pregnancy, some patients do experience temperature increases. In some patients, carboprost tromethamine may cause transient bronchoconstriction.
Metabolism
Metabolized in the lungs and liver. Metabolites are excreted in urine.
Properties of Carboprost tromethamine
| Melting point: | >85°C (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Soluble in water. |
| form | neat |
| form | Solid |
| color | White to Pale Beige |
| Stability: | Hygroscopic |
Safety information for Carboprost tromethamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Carboprost tromethamine
| InChIKey | UMMADZJLZAPZAW-XOWPVRJPSA-N |
Carboprost tromethamine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 58551-69-2 Carboprost tromethamine 98%View Details
58551-69-2 Carboprost tromethamine 98%View Details
58551-69-2 -
 58551-69-2 98%View Details
58551-69-2 98%View Details
58551-69-2 -
 Carboprost Tromethamine 98%View Details
Carboprost Tromethamine 98%View Details
58551-69-2 -
 Carboprost Tromethamine 58551-69-2 98%View Details
Carboprost Tromethamine 58551-69-2 98%View Details
58551-69-2 -
 58551-69-2 98%View Details
58551-69-2 98%View Details
58551-69-2 -
 Carboprost tromethamine CAS 58551-69-2View Details
Carboprost tromethamine CAS 58551-69-2View Details
58551-69-2 -
 Carboprost Tromethamine APIView Details
Carboprost Tromethamine APIView Details
58551-69-2 -
 Carboprost Tromethamine APIView Details
Carboprost Tromethamine APIView Details
58551-69-2
