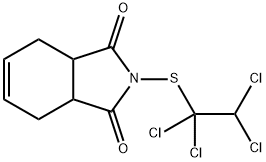
CAPTAFOL
- CAS NO.:2425-06-1
- Empirical Formula: C10H9Cl4NO2S
- Molecular Weight: 349.06
- MDL number: MFCD00041816
- EINECS: 219-363-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is CAPTAFOL?
Description
Captafol appears as white, colourless to pale yellow, or tan (technical-grade) crystals or as a crystalline solid or powder, with a slight characteristic pungent odour. It is practically insoluble in water but is soluble or slightly soluble in most organic solvents. Captafol reacts with bases, acids, acid vapours, and strong oxidisers. Captafol is a broad-spectrum nonsystemic fungicide that is categorised as a phthalimide fungicide based on its tetrahydrophthalimide chemical ring structure (other phthalimide fungicides include captan and folpet). It hydrolyses slowly in aqueous emulsions or suspensions but rapidly in acidic and basic aqueous alkaline media. Captafol will not burn, but when heated to decomposition, it emits toxic fumes, including nitrogen oxides, sulphur oxides, phosgene, and chlorine. Captafol is effective for the control of almost all fungal diseases of plants except powdery mildews and is widely used outside the United States for the control foliage and fruit disease on apples, citrus, tomato, cranberry, potato, coffee, pineapple, peanut, onion, stone fruit, cucumber, blueberry, prune, watermelon, sweet corn, wheat, barley, oilseed rape, leek, and strawberry. It is also used as a seed protectant in cotton, peanuts, and rice. Captafol is also used in the lumber and timber industries to reduce losses from wood rot fungi in logs and wood products. Formulations of captafol include dusts, flowables, wettables, water dispersibles, and aqueous suspensions. Mixed formulations include (captafol +) triadimefon, ethirimol, folpet, halacrinate, propiconazole, and pyrazophos. Captafol is compatible with most plant-protection products, with the exception of alkaline preparations and formulating material.
Chemical properties
yellow to off-white powder
Chemical properties
Captafol is a white crystalline solid.
The Uses of CAPTAFOL
Captafol is used to control a wide range of fungal diseases on many crops.
The Uses of CAPTAFOL
Agricultural fungicide, especially for potatoes.
The Uses of CAPTAFOL
Captafol is a pesticide, belonging to thiophtalimide group. Occupational contact dermatitis was reported in an agricultural worker who had multiple sensitizations.
Definition
ChEBI: A dicarboximide that captan in which the trichloromethyl group is replaced by a 1,1,2,2-tetrachloroethyl group. A broad-spectrum fungicide used to control diseases in fruit and potatoes, it is no longer approved for use in the European Community.
General Description
White crystalline solid with a slight, but pungent odor. Mp: 162°C. Practically insoluble in water. Only slightly soluble in organic solvents. Technical CAPTAFOL is a wettable light tan powder that is used as a fungicide. Inhaled dust irritates the respiratory tract. Irritates skin and damages eyes. Acute oral toxicity in humans is low. Not persistent in the environment (decomposes with a half-life of 11 days in the soil). Highly toxic to fish and other aquatic organisms.
Reactivity Profile
CAPTAFOL is non-flammable but, on heating, may decompose to generate toxic fumes, such as sulfur oxides, hydrogen sulfide, hydrochloric acid, and phosgene. Stable at room temperature when dry but readily hydrolysed, especially in an alkaline environment. CAPTAFOL and mixtures containing high concentrations of CAPTAFOL may react violently with alkali. Incompatible with acids, acid chlorides, acid anhydrides, and strong oxidizing agents. Sulfhydryl compounds such as glutathione and cysteine cause a rapid chemical decomposition.
Hazard
Absorbed by skin. Probable carcinogen.
Agricultural Uses
Fungicide: Captafol is a General Use Pesticide and used for the control of practically all forms of fungal diseases except powdery mildew. It is also used as a seed protectorant on cotton, rice and peanut crops. Not registered for use in the U.S. or in EU countries. There are 20 global suppliers.
Trade name
CAPTATOL®; CAPTOFOL®; CRISFOLATAN®; DIFOLATAN®[C]; DIFOCAP®[C]; DIFOSAN®; FOLCID®; HAIPEN®; KENOFOL®; MERPAFOL®; ORTHO® 5865[C]; PILLARTAN®; SANSEAL®; SANSPOR®; SANTAR-SM®; SULFONIMIDE®; SULPHEIMIDE®
Contact allergens
Captafol is a pesticide, belonging to thiophthalimide group. Occupational contact dermatitis was reported in an agricultural worker who had multiple sensitizations
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A fungicide. When heated to decomposition it emits very toxic fumes of Cl-, NO,, and SOx
Potential Exposure
Captafol is a thiophthalimide fungicide. Those engaged in the manufacture, formulation, and application of this fungicide. Captafol is not currently registered for use on field crops or stored produce in the United States.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPRif heart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Captafol is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and supporting data on mechanisms of carcinogenesis.
Environmental Fate
The primary toxicity following captafol exposure probably occurs through a hypersensitivity mechanism. Most experiments suggest captafol to be DNA active.
Metabolic pathway
Captafol contains an unstable tetrachloroethylthio (sulfenyl) moiety that has been shown to undergo rapid hydrolytic and metabolic degradation to tetrahydrophthalimide (2). By analogy with captan, presumably the tetrachloroethylthio moiety can be transferred to the sulfur atoms of thiols such as cysteine and glutathone. Thus in the presence of thiols such as glutathione, captafol is probably cleaved at the N-S bond to form thiophosgene (3) and other gaseous products such as hydrogen sulfide, hydrogen chloride and carbonyl sulfide. Thiophosgene is rapidly hydrolysed by water. The tetrachloroethylthio group and thiophosgene are believed to be intermediates in the formation of thiazolidine-2- thione-6carboxylic acid (4) which is an addition product with cysteine. A thiazolidine derivative of glutathione is also formed (5). Biotransformation of captafol in mammals generates primarily thiophosgene (3) and tetrahydrophthalimide (2). Tetrahydrophthalimide (2) and various of its derivatives are excreted in the urine. There were no reports of 2-thiazolidinethione-4-carboxylic acid (4) in the urine.
storage
Color Code—Green: General storage may be used.Prior to working with captafol you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from heat, acids,acid fumes, or strong oxidizers (such as peroxides, chlorates, perchlorates, nitrates and permanganates), since violent reactions occur. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN 2773 Phthalimide derivative pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Degradation
Captafol is hydrolysed rapidly in acidic and alkaline conditions. It
decomposes slowly at its melting point of 161 °C (PM). Captafol is
decomposed by base-catalysed hydrolysis with half-lives of 77.8, 6.54 and
0.72 hours at pH 3,7 and 8, respectively (Kim et al., 1997)
In a study of aqueous photodegradation, a solution (MeCN/H2O 9:l)
of unlabelled captafol (10 g l-1) was exposed to UV light for 4 days. The
reaction tube was encircled by low pressure Hg lamps giving more than
85% of total radiation at 253.7 nm. Pure nitrogen was bubbled through the
solutions. Photo-oxidation studies were done similarly except that oxygen
was bubbled through the solution. In further experiments, irradiation was
by visible light from a tungsten lamp and again oxygen was bubbled
through the solution. The outlet gases from the UV study were trapped in
sodium hydroxide solution and analysed by GC-MS. The main photoproduct
was usually tetrahydrophthalimide (2). Analysis was by chromatography
and IR and NMR spectroscopy. Photolysis under nitrogen gave
2 in 72% yield with elemental sulfur and HCl as the only products other
than some parent captafol. Photooxidation of captafol gave tetrahydrophthalimide
(2) in 78% yield together with sulfur dioxide, carbon dioxide,
hydrogen chloride and some unreacted captafol. Oxidation of captafol
in the presence of visible light together with Rose Bengal as a photosensitiser
also gave a high yield of tetrahydrophthalimide (2) and the
other products obtained from photo-oxidation. Although the mechanisms
of reaction were not studied, it is possible that sulfoxidised intermediates
could be involved in reactions with singlet oxygen. It was surprising that the cyclohexene moiety did not react with singlet oxygen and that
products of ring oxidation were not observed (Crank and Mursyidi, 1992).
Toxicity evaluation
Captafol is not persistent in the environment. Captafol is stable under ordinary environmental conditions and rapidly degrades in soil, the rate of degradation being a function of soil type and pesticide concentration. It does not leach from basic soils and is unlikely to contaminate groundwater. Captafol sprayed on most crops has a half-life of less than 5 days. Captafol and/or its metabolites and degradation products are readily absorbed by roots and shoots of plants. If released to air, an extrapolated vapor pressure of 8.27×10-9 mm Hg at 25°C indicates captafol will exist solely in the particulate phase in the ambient atmosphere. Particulate-phase captafol will be removed from the atmosphere by wet and dry deposition. If released to soil, captafol is expected to have slight mobility based on Koc values of 2073 and 2120. Volatilization from moist soil surfaces is not expected to be an important fate process based on a Henry’s Law constant of 2.7×10-9 atm-cu m mol-1. In a laboratory setting, the biodegradation half-life of captafol in three soils was found in the range of 23–55 days. The overall half-life of captafol in soil is about 11 days, independent of soil type or initial concentration. If released into water, captafol is expected to adsorb to suspended solids and sediment based on the Koc. Volatilization from water surfaces is not expected to be an important fate process based on this compound’s estimated Henry’s Law constant. An estimated bioconcentration factor of 170 suggests the potential for bioconcentration in aquatic organisms is high, provided the compound is not altered physically or chemically after being released to the environment. The half-lives for the hydrolysis of Difolatan at pH 3.0, 7.0, and 8.0 were 77.8, 6.54, and 0.72 h, respectively. Hydrolysis is likely to be the predominant pathway of degradation in the aquatic environment.
Incompatibilities
Reacts violently with bases, causing fire and explosion hazard. Not compatible with strong acids or acid vapor, oxidizers. Strong alkaline conditions contribute to instability. Attacks some metals.
Waste Disposal
Hydrolysis.
Properties of CAPTAFOL
| Melting point: | 160-161° |
| Boiling point: | 365.7±52.0 °C(Predicted) |
| Density | 1.4682 (rough estimate) |
| vapor pressure | 1.1 x 10-6 Pa (20 °C) |
| refractive index | 1.6000 (estimate) |
| Flash point: | >100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Sparingly), Dichloromethane (Very Slightly), Methanol (Slightly) |
| form | Solid |
| Water Solubility | 1.4 mg l-1 (20 °C) |
| pka | -2.67±0.20(Predicted) |
| color | White to Off-White |
| Merck | 13,1777 |
| IARC | 2A (Vol. 53) 1991 |
| EPA Substance Registry System | Captafol (2425-06-1) |
Safety information for CAPTAFOL
Computed Descriptors for CAPTAFOL
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Captafol CAS 2425-06-1View Details
Captafol CAS 2425-06-1View Details
2425-06-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8