1,1,2,2-Tetrachloroethane
Synonym(s):1,1,2,2-Tetrachloroethane;Acetylene tetrachloride
- CAS NO.:79-34-5
- Empirical Formula: C2H2Cl4
- Molecular Weight: 167.85
- MDL number: MFCD00000848
- EINECS: 201-197-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
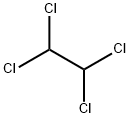
What is 1,1,2,2-Tetrachloroethane?
Description
1,1,2,2-Tetrachloroethane (tetrachloroethane) is a volatile, synthetic, colorless to pale-yellow dense liquid with a pungent, chloroform-like odor that is soluble in water and most organic solvents. There are no known natural sources of tetrachloroethane. Tetrachloroethane is a chemical intermediate in the production of a variety of other common chemicals. In the past, the major use for tetrachloroethane was in the production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene. With the development of new processes for manufacturing chlorinated ethylenes and the availability of less-toxic solvents, the production of tetrachloroethane as a commercial endproduct in the United States and Canada has steadily declined since the late 1960s and the production ceased by the early 1990s. Although at one time it was used as an insecticide, fumigant, and weed killer, it presently is not registered for any of these purposes. Its registration as an insect repellent was canceled by the Environmental Protection Agency (EPA) in the late 1970s.
Chemical properties
colourless to light yellow liquid with a chloroform-like
Chemical properties
Tetrachloroethane is a heavy, volatile colorless to light yellow liquid. It has a sweetish, chloroform-like odor. The Odor Threshold is 0.5 ppm in water and 1.5 ppm in air.
Physical properties
Colorless to pale yellow liquid with a sweet, chloroform-like odor. A detection odor threshold concentration of 50 mg/m3 (7.3 ppmv) was experimentally determined by Dravnieks (1974).
The Uses of 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane, once used as a solvent for cleaning and extraction processes, is still used to some extent as a chemical intermediate. Present usage is quite limited because less toxic solvents are available.
The Uses of 1,1,2,2-Tetrachloroethane
Nonflammable solvent for fats, oils, waxes, resins, cellulose acetate, rubber, copal, phosphorus, sulfur. As solvent in certain types of Friedel-Crafts reactions or phthalic anhydride condensations. In the manufacture of paint, varnish, and rust removers. In soil sterilization and weed killer and insecticide formulations. In the determination of theobromine in cacao. As immersion fluid in crystallography. In the biological laboratory to produce pathological changes in gastrointestinal tract, liver, and kidneys. Intermediate in the manufacture of trichloroethylene and other chlorinated hydrocarbons having two carbon atoms.
The Uses of 1,1,2,2-Tetrachloroethane
Intermediate in the production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene; previously used as a solvent, insecticide and fumigant.
Definition
ChEBI: A member of the class of chloroethanes that is ethane substituted by chloro groups at positions 1, 1, 2 and 2.
General Description
Colorless to pale yellow liquid with a sweet odor. Sinks in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,2,2-Tetrachloroethane may be incompatible with strong oxidizing and reducing agents. Also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Decomposed by heat and UV light, forming phosgene and HCl; reacts violently with finely dispersed metals [Handling Chemicals Safely 1980. p. 886].
Hazard
Toxic by ingestion, inhalation, skin absorption. Questionable carcinogen.
Health Hazard
Compound is a powerful narcotic and liver poison; may also cause changes in blood composition and neurological disturbances. Repeated exposure by inhalation can be fatal. Ingestion causes vomiting, diarrhea, severe mucosal injury, liver necrosis, cyanosis, unconsciousness, loss of reflexes, and death. Contact with eyes causes irritation and lachrymation. Can be absorbed through the skin and may produce severe skin lesions.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride vapor may form in fire.
Safety Profile
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Poison by inhalation, ingestion, and intraperitoneal routes. Moderately toxic by several other routes. Mutation data reported. Human central nervous system effects by ingestion and inhalation: general anesthesia, somnolence, hallucinations, and distorted perceptions. Considered the most toxic of the common chlorinated hydrocarbons. Considered to be a very severe industrial hazard and its use has been restricted or even forbidden in certain countries. It is not an inert solvent. Reacts violently with N2O4,2,4dinitrophenyl disulfide, and on contact with sodium or potassium. When heated in contact with solid potassium hydroxide, spontaneously flammable chloroor dichloroacetylene gas is evolved. Any water can cause appreciable hydrolysis, even at room temperature, and both hydrolysis and oxidation become comparatively rapid above 110'. When heated to decomposition it emits toxic fumes of Cl-. A strong irritant of eyes and mucous membranes. A concentration of 3 ppm produces a detectable odor, thus an initial produces a detectable odor, thus an initial warning effect. Its narcotic action is stronger than that of chloroform, but, because of its low volatility, narcosis is less severe and much less common in industrial poisoning than in the case of other chlorinated hydrocarbons. The toxic action of this material is chiefly on the liver, where it produces acute yellow atrophy and cirrhosis. Fatty degeneration of the kidneys and heart, hemorrhage into the lungs and serous membranes, and edema of the brain have also been found in fatal cases. Some reports indicate a toxic action on the central nervous system with changes in the brain and in the peripheral nerves. The effect on the blood is one of hemolysis with appearance of young cells in the circulation and a monocptosis. Due to its solvent action on the natural skin oils, dermatitis is not uncommon. The initial symptoms resulting from exposure to the vapor are lachrymation, salivation, and irritation of the nose and throat. Continued exposure to high concentrations results in restlessness, dizziness, nausea, vomiting, and narcosis. The latter, however, is rare in industry. More commonly, exposure is less severe and most complaints are vague and related to the digestive and nervous systems. The patient's symptoms gradually progress to a more serious illness with development of toxic jaundice, liver tenderness, etc., and possibly albuminuria and edema. With serious liver damage, the jaundice increases and toxic symptoms appear, with somnolence, delirium, convulsions, and coma usually precedmg death. See also ACETYLENE COMPOUNDS and CHLORIDES.
Potential Exposure
Tetrachloroethane is used as an intermediate in the trichloroethylene production from acetylene and as a solvent; as a dry cleaning agent; as a fumigant; in cement; and in lacquers. It is used in the manufacture of artificial silk, artificial leather, and artificial pearls. Recently, its use as a solvent has declined due to replacement by less toxic compounds. It is also used in the estimation of water content in tobacco and many drugs, and as a solvent for chromium chloride impregnation of furs.
Carcinogenicity
The EPA has classified this
material as “likely to be carcinogenic to humans” based on data from an oral cancer bioassay in male and female
Osborne–Mendel rats and B6C3F1 mice. In mice, a
significant increase in the incidence of hepatoceullar carcinomas
in both genders was observed. Male Osborne–Mendel
rats showed increased incidence of hepatocellular carcinomas,
which is a rare tumor in this strain.
The National Cancer Institute has included 1,1,2,2-
tetrachloroethane in their bioassay series using rats and mice.
Their summary states that the time-weighted average doses
(by gavage) were 108 and 62 mg/kg/day for male rats, 76 and
43 mg/kg/day for female rats, and 282 and 142 mg/kg/day for
all mice. There was a highly significant positive dose-related
trend in the incidence of hepatocellular carcinoma in mice of
both sexes. No statistically significant incidence of neoplastic
lesions was observed in male or female rats. However, two
hepatocellular carcinomas and one neoplastic nodule, which
are rare tumors in the male Osborne–Mendel rat, were
observed in high-dose males. Under the conditions of this
bioassay, orally administered 1,1,2,2-tetrachloroethane was
a liver carcinogen in B6C3Fl mice of both sexes.
The proposed metabolic pathway involves the production
of dichloroacetic acid, which was identified as the
major urinary metabolite in treated mice. Other pathways
involve formation of trichloroethylene via dehydrochlorination
or tetrachloroethylene via oxidation. Free radicals may
also be formed.
From the NCI study, a oral slope factor (OSF) of 0.2
per mg/kg/day was developed by the EPA. No inhalation unit
risk (IUR) was determined by the EPA because of absence of
data from inhalation exposure.
Environmental Fate
Biological. Monodechlorination by microbes under laboratory conditions produced 1,1,2-
trichloroethane (Smith and Dragun, 1984). In a static-culture-flask screening test, 1,1,2,2-
tetrachloroethane (5 and 10 mg/L) was statically incubated in the dark at 25 °C with yeast extract
and settled domestic wastewater inoculum. No significant degradation was observed after 28 d of
incubation (Tabak et al., 1981).
Chemical/Physical. In an aqueous solution containing 0.100 M phosphate-buffered distilled
water, 1,1,2,2-tetrachloroethane was abiotically transformed to 1,1,2-trichloroethane. This reaction
was investigated within a temperature range of 30 to 95 °C at various pHs (5 to 9) (Cooper et al.,
1987). Abiotic dehydrohalogenation of 1,1,2,2-tetrachloroethane yielded trichloroethylene (Vogel
et al., 1987) and HCl (Kollig, 1993). The half-life for this reaction at 20 °C was reported to be 0.8
yr (Vogel et al., 1987). Under alkaline conditions, 1,1,2,2-tetrachloroethane dehydrohalogenated
to trichloroethylene. The reported hydrolysis half-life of 1,1,2,2-tetrachloroethane in water at 25
°C and pH 7 is 146 d (Jeffers et al., 1989).
The evaporation half-life of 1,1,2,2-tetrachloroethane (1 mg/L) from water at 25 °C using a
shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 55.2 min (Dilling,
1977).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities
were 11, 4.5, 1.9, and 0.8 mg/g, respectively (Dobbs and Cohen, 1980).
Shipping
UN1702 Tetrachloroethane or 1,1,2,2Tetrachloroe thane, Hazard Class: 6.1; Labels: 6.1Poisonous materials.
Purification Methods
Stir the ethane, on a steam-bath, with conc H2SO4 until a fresh portion of acid remains colourless. The organic phase is then separated, distilled in steam, dried (CaCl2 or K2CO3), and fractionally distilled in a vacuum. [Beilstein 1 IV 144.]
Toxicity evaluation
Metabolism of tetrachloroethane to reactive products plays
a key role in its toxicity. Both nuclear and microsomal cytochrome
P450 enzymes have been implicated in the metabolism
of the compound, possibly releasing aldehydes, alkenes,
acids, and free radicals that may react with biological tissues.
Therefore, because of high metabolic activity of the liver, the
formation of active metabolites is a likely mechanism for the
toxicity tetrachloroethane. Hence, tetrachloroethane metabolism
could result in the reductive formation of radical products,
leading to the stimulation of lipid peroxidation resulting in
hepatotoxic effects, as noted in carbon tetrachloride, a structurally
related chlorinated alkane. Both dichloroacetic and trichloroacetic
acids are known to cause proliferation of
peroxisomes.
The mechanism of neurological toxicity of tetrachloroethane
has not been well characterized. Studies of similar
compounds suggest that the parent compound itself may be
the causative agent. This property allows interference with
neural membrane function, bringing about central nervous
system depression, behavioral changes, and anesthesia.
The mechanisms by which tetrachloroethane produces
carcinogenic effects are incompletely characterized. Tetrachloroethane
has been shown to bind to DNA in the liver and several
other organs in rats and mice, which may contribute to the
carcinogenic process. Studies indicate that there may be initiating
and promoting activities when tetrachloroethane is metabolized,
possibly by cytochrome P450 enzymes producing urinary
metabolites such as dichloroacetic acid, trichloroacetic acid,
trichloroethylene, and tetrachloroethylene. Studies of chronic exposure of rats and mice to these specific metabolites revealed
hepatic tumors in male and female mice. Although it is possible
that the carcinogenicity of tetrachloroethane involves metabolism
with these compounds, there is no direct evidence linking
one or more metabolites to its carcinogenic effects. Tetrachloroethane
may be metabolized to form free radicals, which
can in turn covalently bind to tissues, including DNA.
Incompatibilities
Violent reaction with chemically active metals; strong caustics; strong acids; especially fuming sulfuric acid. Degrades slowly when exposed to air. Attacks plastic and rubber.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of 1,1,2,2-Tetrachloroethane
| Melting point: | -43 °C |
| Boiling point: | 147 °C(lit.) |
| Density | 1.586 g/mL at 25 °C(lit.) |
| vapor density | 5.8 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 142-146°C |
| storage temp. | Store below +30°C. |
| solubility | 2830g/l |
| form | Liquid |
| color | slightly green-yellow |
| Water Solubility | 0.3 g/100 mL (25 ºC) |
| Merck | 14,9189 |
| BRN | 969206 |
| Henry's Law Constant | 6.22 at 30 °C (headspace-GC, Sanz et al., 1997) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: TWA 1 ppm (7 mg/m3), IDLH
100 ppm; OSHA PEL: TWA 5 ppm (35 mg/m3); ACGIH TLV: TWA 1 ppm (adopted). |
| Dielectric constant | 8.4199999999999999 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. Reacts violently with sodium, potassium, nitrates, 2,4-dinitrophenyl disulphide. |
| CAS DataBase Reference | 79-34-5(CAS DataBase Reference) |
| IARC | 2B (Vol. 20, Sup 7, 71, 106) 2014 |
| NIST Chemistry Reference | Ethane, 1,1,2,2-tetrachloro-(79-34-5) |
| EPA Substance Registry System | 1,1,2,2-Tetrachloroethane (79-34-5) |
Safety information for 1,1,2,2-Tetrachloroethane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane manufacturer
JSK Chemicals
Suvidhinath Laboratories
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![TERT-BUTYL 4,4,5,5-TETRACHLOROSPIRO[2.2]PENTANE-1-CARBOXYLATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB7487008.gif)
![1,1,2,2-TETRACHLOROETHANE [1,2-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB3227468.gif)
You may like
-
 1,1,2,2-Tetrachloroethane extrapure AR CAS 79-34-5View Details
1,1,2,2-Tetrachloroethane extrapure AR CAS 79-34-5View Details
79-34-5 -
 Acetylene tetrachloride, puriss CAS 79-34-5View Details
Acetylene tetrachloride, puriss CAS 79-34-5View Details
79-34-5 -
 1,1,2,2-Tetrachloroethane CAS 79-34-5View Details
1,1,2,2-Tetrachloroethane CAS 79-34-5View Details
79-34-5 -
 1,1,2,2-Tetrachloroethane, 98.5% CAS 79-34-5View Details
1,1,2,2-Tetrachloroethane, 98.5% CAS 79-34-5View Details
79-34-5 -
 1,1,2,2-Tetrachloroethane, GR 99%+ CAS 79-34-5View Details
1,1,2,2-Tetrachloroethane, GR 99%+ CAS 79-34-5View Details
79-34-5 -
 1,1,2,2-Tetrachloroethane CAS 79-34-5View Details
1,1,2,2-Tetrachloroethane CAS 79-34-5View Details
79-34-5 -
 Liquid 1,1,2,2 Tetrachloro Ethane, Grade Standard: Analytical Grade, Bio-Tech GradeView Details
Liquid 1,1,2,2 Tetrachloro Ethane, Grade Standard: Analytical Grade, Bio-Tech GradeView Details
79-34-5 -
 1,1,2,2-Tetrachloroethane, Grade: Analytical GradeView Details
1,1,2,2-Tetrachloroethane, Grade: Analytical GradeView Details
79-34-5
