Ethoprophos
Synonym(s):O-Ethyl S,S-dipropyl phosphorodithioate;Ethoprofos;Ethoprop
- CAS NO.:13194-48-4
- Empirical Formula: C8H19O2PS2
- Molecular Weight: 242.34
- MDL number: MFCD00055333
- EINECS: 236-152-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
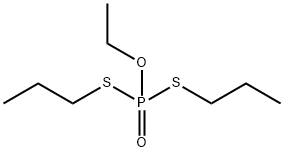
What is Ethoprophos?
Description
Introduced in the 1960s, ethoprop is a nonsystemic insecticide/nematicide. The mobility of ethoprop in soil and its half-life are strongly dependent on soil organic matter (21). It is not known to be carcinogenic and is available as granules or emulsifiable concentrates.
Chemical properties
Clear Colorless Oil
Chemical properties
Ethoprophos is a pale yellow liquid.
The Uses of Ethoprophos
Ethoprophos is an organophosphate non-systemic nematicide and soil insecticide. Ethoprophos is an acetylcholinesterase (AChE) inhibitor.
The Uses of Ethoprophos
Insecticide; nematocide.
The Uses of Ethoprophos
Nonsystemic, nonfumigant nematocide and soil insecticide for control of insects in ornamentals, potatoes, sweet potatoes, tomatoes, strawberries, bananas, pineapples, sugar cane, turf and many other crops.
The Uses of Ethoprophos
Ethoprophos is used to control plant parasitic nematodes and soil insects.
Definition
ChEBI: Ethoprophos is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an agrochemical and an antinematodal drug.
General Description
Ethorop is one of a family of organophosphorus pesticides. Mocap is combustible though Mocap may require some effort to ignite. Mocap is very toxic by skin absorption and inhalation. Mocap may or may not be water soluble.
Air & Water Reactions
Hydrolyzed in alkali.
Reactivity Profile
Organothiophosphates, such as Mocap, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Mocap is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150 lb. person. It is a cholinesterase inhibitor which affects the nervous system.
Fire Hazard
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) Container may explode in heat of fire. Fire and runoff from fire control water may produce irritating or poisonous gases. Stable in water. Hydrolyzed in alkali.
Agricultural Uses
Nematicide, Insecticide: Ethoprop is used as a pre-plant soil application to control wireworms and nematodes in potatoes, sugar cane, sweet potatoes, and tobacco, with lesser usage on corn (field and sweet), beans (lima and snap), cucumbers, and cabbage. In addition, it is used to treat pineapples, bananas, and plantains, as well as fieldgrown ornamentals and non-bearing citrus trees, and commercial turf. Roughly 60% of ethoprop is applied to potatoes.
Trade name
AI3-27318®; CASWELL No. 434C®; JOLT®[C]; MENAP®; MOBIL V-C 9-104®; MOCAP®[C]; MOCAP 10G®[C]; PHOSETHOPROP®; ROVOKIL®; V-C 9-104®[C]; V-C CHEMICAL V-C 9-104®[C]; VIRGINIA-CAROLINA VC 9-104®
Safety Profile
Poison by ingestion and skin contact. A cholinesterase inhibitor type of insecticide. When heated to decomposition it emits very toxic fumes of POx and SOx. See also PARATHION.
Potential Exposure
A potential danger to those involved in the manufacture, formulation and application of this nematocide and soil insecticide
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If this chemicalhas been inhaled, remove from exposure, begin rescuebreathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Remove and isolatecontaminated clothing and shoes at the site. Keep victimquiet and maintain normal body temperature. Effects may bedelayed; keep victim under observation
Carcinogenicity
In a combined chronic feeding/ carcinogenicity study, when rats were fed diets with 0, 1, 10, or 100 ppm ethoprop in the diet for 24 months (equivalent to 0, 0.041, 0.40, or 4.19 mg/kg/day (males); 0, 0.052, 0.51, or 5.12 mg/kg/day (females)), adrenal gland malignant pheochromocytomas were increased in males and thyroid C-cell carcinomas were increased slightly in males . When ethoprop was administered in the diet to mice for 104 weeks at 0, 0.2, 2.0, or 30 ppm (males: 0, 0.026, 0.254, or 3.96 mg/ kg/day; females: 0, 0.032, 0.318, or 4.9 mg/kg/day), survival was unaffected at any dose level and no statistically significant dose-related incidence of tumors were seen in males or females.
Environmental Fate
Soil. The reported half-life in humus-containing soil (pH 4.5) and a sandy loam (pH
7.2–7.3) are 87 and 14–28 days, respectively (Hartley and Kidd, 1987). The rate of
degradation increased in soils that had been treated annually four times (Smelt et al., 1987).
Chemical/Physical. Emits toxic fumes of phosphorus and sulfur oxides when heated
to decomposition (Sax and Lewis, 1987).
Metabolic pathway
Ethoprophos is a soil-acting insecticide and nematicide which is generally incorporated into the soil in the form of a granular formulation. Volatilisation is a factor involved in the loss and movement of the compound in the environment. The major route of degradation of ethoprophos in both plants and mammals is via hydrolysis to give O-ethyl S-propyl phosphorothioate through loss of propanethiol. Propanethiol has been shown to react as a nucleophile to yield ethyl propyl sulfide through attack on the phosphorus atom and dipropyl disulfide through attack on the sulfur atom of ethoprophos. The sulfide metabolites are further metabolised via oxidation to sulfoxides and sulfones. In mammals, an additional important route of detoxification is through de-ethylation via glutathione- S-alkyl transferase, a route common to many organophosphorus insecticides.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area. Where possible, automatically pump liquidfrom drums or other storage containers to processcontainers.
Shipping
UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Degradation
Ethoprophos is very stable in neutral and weakly acidic media but it is hydrolysed rapidly in alkaline solutions (PM). The DT50 of ethoprophos in 0.05M NaOH solution at 25 °C was 35 min and the hydrolysis product was identified as O-ethyl S-propyl phosphorothioate (2), indicating the greater susceptibility of the P-S bond to hydrolysis over the P-O bond. There was no measurable hydrolysis in 0.1M HCl after 1 hour (Menzer et al., 1971). The route of the base-catalysed hydrolysis of ethoprophos is shown in Scheme 1.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Strong oxidizers may cause release of toxic phosphorus oxides. Organophosphates, in the presence of strong reducing agents such as hydrides, may form highly toxic and flammable phosphine gas. Keep away from alkaline materials
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office
Properties of Ethoprophos
| Melting point: | -13 °C |
| Boiling point: | 86-91°C |
| Density | d420 1.094 |
| vapor pressure | 4.65 x 10-2 Pa (26 °C) |
| Flash point: | 100 °C |
| storage temp. | APPROX 4°C
|
| solubility | DMF: 5mg/mL; DMSO: 5mg/mL; Ethanol: 1.6mg/mL |
| form | neat |
| Water Solubility | 700 mg l-1 (20 °C) |
| Merck | 13,3782 |
| BRN | 8139788 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 13194-48-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethoprophos(13194-48-4) |
| EPA Substance Registry System | Ethoprop (13194-48-4) |
Safety information for Ethoprophos
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ethoprophos
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Ethoprophos CAS 13194-48-4View Details
Ethoprophos CAS 13194-48-4View Details
13194-48-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8