Carbofuran
Synonym(s):2,3-Dihydro-2,2-dimethyl-7-benzofuranol N-methylcarbamate
- CAS NO.:1563-66-2
- Empirical Formula: C12H15NO3
- Molecular Weight: 221.25
- MDL number: MFCD00041819
- EINECS: 216-353-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
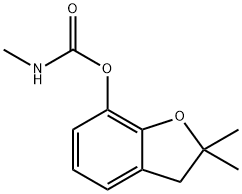
What is Carbofuran?
Description
Carbofuran is another systemic insecticidal/nematicidal carbamate available in granular and liquid formulations. Because use of carbofuran granules was associated with bird kills, the U.S. Environmental Protection Agency (EPA) prohibited the use of carbofuran granules in 1994.
Description
Carbofuran is a carbamate pesticide that acts as a cholinesterase inhibitor. A Dutch patent first described it for this use in 1964. Europe has banned carbofuran’s use because it is extremely toxic to humans and wildlife. In July, the U.S. Environmental Protection Agency announced that it intends to seek a ban on all uses of carbofuran in the United States.
Chemical properties
Carbofuran is a broad-spectrum carbamate insecticide and nematicide. It is an odorless, white crystalline solid. On heating, it breaks down and can release toxic fumes, and irritating or poisonous gases. It is sparingly soluble in water, but very soluble in acetone, acetonitrile, benzene, and cyclohexone. The liquid formulations of carbofuran are classifi ed as RUPs because of their acute oral and inhalation toxicity to humans. Granular formulations are also classifi ed as an RUP. In fact, carbofuran was fi rst registered in the United States in 1969 and classifi ed as an RUP. Exposure to heat breaks down carbofuran, with the release of toxic fumes. Carbofuran is used for the control of soil-dwelling and foliar-feeding insects. It is also used for the control of aphids, thrips, and nematodes that attack vegetables, ornamental plants, crops of sunfl ower, potatoes, peanuts, soybeans, sugar cane, cotton, rice, and a variety of other crops
The Uses of Carbofuran
Carbofuran is used to control soil-dwelling insect pests and nematodes in a wide range of crops.
The Uses of Carbofuran
Cholinesterase inhibitor. Use as systemic insecticide, acaricide, nematocide.
The Uses of Carbofuran
Broad-spectrum, systemic insecticide, nematocide and acaricide applied in soil to control soil insects and nematodes or on foliage to control insects and mites.
What are the applications of Application
Carbofuran is AChE and BChE (cholinesterase) inhibitor
Definition
ChEBI: Carbofuran is a carbamate ester and a member of 1-benzofurans. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a carbamate insecticide, an EC 3.1.1.8 (cholinesterase) inhibitor, an acaricide, an agrochemical, an avicide and a nematicide.
General Description
Carbofuran is an odorless white crystalline solid. Contact with skin may burn skin and eyes. When exposed to heat or flames Carbofuran may emit toxic oxides of nitrogen. Carbofuran is toxic by inhalation, skin contact, and/ or ingestion. Carbofuran is used as a pesticide.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Carbofuran is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. Carbofuran is unstable in an alkaline media. .
Health Hazard
The acute oral LD50 of carbofuran to male and female rats is about 8 mg/kg, while the acute dermal LD50 for rats is more than 3000 mg/kg. Carbofuran is mildly irritating to the eyes and skin of rabbits. The acute inhalation toxicity (LC50, 4 h) is 0.075 mg/L for rats. As with other carbamate compounds, carbofuran’s cholinesterase-inhibiting effect is short term and reversible. The symptoms of carbofuran poisoning include, but are not limited to, nausea, vomiting, abdominal cramps, sweating, diarrhea, excessive salivation, weakness, imbalance, blurring of vision, breathing diffi culty, increased blood pressure, and incontinence. Death may result at high doses from respiratory system failure associated with carbofuran exposure. Complete recovery from an acute poisoning by carbofuran, with no long-term health effects, is possible if exposure ceases and the victim has time to regain his or her normal level of cholinesterase and to recover from symptoms. Reports have indicated that risks from exposure to carbofuran are especially high among occupational workers and general public suffering with asthma, diabetes, cardiovascular disease, gastrointestinal or urogenital tracts disturbances. The available studies indicate carbofuran is unlikely to cause reproductive effects in humans at expected exposure levels. Studies indicate carbofuran is not teratogenic. No signifi cant teratogenic effects have been found in the offspring of rats given carbofuran (3 mg/kg/day) on days 5 to 19 of gestation.
Fire Hazard
May release nitrogen oxides. Containers may explode in heat of fire. Avoid alkalies. Stable under neutral or acid conditions.
Agricultural Uses
Insecticide, Acaricide, Nematicide: Carbofuran is a broad-spectrum carbamate pesticide that kills insects, mites, and nematodes on contact or after ingestion. It is used against soil and foliar pests of field, fruit, vegetable, and forest crops. Carbofuran, granule form, is banned in the U.S. A U.S. EPA restricted Use Pesticide (RUP). Not approved for use in EU countries. There are 40 global suppliers.. According to the Ecological Incident Investigation System, carbofuran has been responsible for more avian deaths than any other pesticide.
Trade name
A13-27164®; AU'ULTRAMICIN®; BAY 704143®; BAY 78537®; BRIFUR®; CARBODAN®; CARBOSIP 5G®; CRISFURAN®; CURETERR®; CHINUFUR®; D 1221®; DIAFURAN®; FMC 10242®; FURACARB®; FURADAN®; FURAN®; FURODAN®; KENFURAN®; KENOFURAN®; NEX®; NIA10242; NIAGARA 10242; NIAGARA NIA-10242; PILLARFURAN®; RAMPART®; YALTOX®
Contact allergens
It is a pesticide with insecticide properties, of the carbamate group. It was implicated as a sensitizer in two farmers
Safety Profile
to decomposition it emits toxic fumes of
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of this insecticide, acaricide, and nematocide.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Effects may be delayed; keep victim under observation.
Environmental Fate
Carbofuran is soluble in water and is moderately persistent in soil. Its half-life is 30–120 days. It enters surface water as a result of runoff from treated fields and enters groundwater by leaching of treated crops. If released to soil, degradation occurs by chemical hydrolysis and biodegradation. The persistence of carbofuran in the soil increases as the clay and organic matter content of the soil increase, and as the pH and moisture content of soil decrease. Chemical hydrolysis occurs more rapidly in alkaline soil as compared to neutral or acidic soils. Carbofuran is likely to leach to groundwater in soils with low organic content. Volatilization from soil is not expected to be significant, although some evaporation from plants may occur. If released to water, carbofuran degrades by hydrolysis under alkaline conditions and by biodegradation. Aquatic volatilization, adsorption, and bioconcentration are not expected to be important.
Metabolic pathway
The fate of carbofuran has been investigated in soils, plants, mammals, birds, fish and insects. Metabolic pathways include hydrolysis, oxidation (ring and N-methyl hydroxylation) and conjugation. The metabolism of carbofuran has been extensively reviewed by Schlagbauer and Schlagbauer (1972) and Kuhr and Dorough (1976). Metabolism in economic animals was reviewed by Akhtar (1985). Consequently the many primary publications are not usually cited.
Metabolism
Carbofuran (1) is degraded by hydrolysis and oxidation in soil before ultimate mineralization . The rate of hydrolysis in soils is slightly higher under flooded than under nonflooded conditions. Products depend on the soil type and the prevalence of aerobic or anaerobic conditions, and it was also reported that carbofuran did not degrade under anaerobic conditions. The products, 3-hydroxycarbofuran (2) and 3-ketocarbofuran (3), have been isolated from soil extracts after incubation with carbofuran. The phenol (7) was identified as a major product in several studies. The products are further degraded and bound to soil organic matter. Enhanced degradation may follow repeated applications of carbofuran to soils, and bacterial cultures capable of rapidly degrading carbofuran have been obtained from treated soils.
Storage
Carbofuran should be stored in a cool, dry, well-ventilated place, in their original containers only. It should not be kept stored or used near heat, open flame, or hot surfaces
Shipping
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN 2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name RequiredUN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Degradation
Carbofuran (1) is very stable in weakly acidic media and has a DT50 of
<1 year at pH 4 (22°C). It is stable in neutral media but unstable in
basic conditions (PM). Carbofuran was hydrolysed to the phenol (5) with
a half-life of 67 minutes at 37.5 °C at pH 9.5. 3-Ketocarbofuran (3)
and N-hydroxymethylcarbofuran (4) (Scheme 2) were hydrolysed faster
than carbofuran in alkaline solution (Metcalf et al., 1968). Unlabelled
carbofuran was dissolved in water and irradiated by sunlight in India
for 30 days. Samples were extracted and analysed by GC and TLC.
Products were 3-ketocarbofuran (3) and the 4-hydroxycarbofuran phenol
(8). (Raha and Das, 1990). Solid carbofuran was applied to glass plates
and irradiated with fluorescent light or sunlight. 3-Hydroxycarbofuran
(2) was detected after 2 days; 3-ketocarbofuran (3) was not detected
(Metcalf et al., 1968). These pathways are illustrated in Scheme 1.
Toxicity evaluation
Carbofuran undergoes hydrolytic and oxidative processes in mammals. In rats, about 72% of the administered dose was eliminated in the urine within 24 hours as conjugated metabolites, mainly as conjugates of the 3- ketocarbofuran phenol (5). The products of metabolism in plants are 3-hydroxycarbofuran (4) and 3-ketocarbofuran phenol (5).
Incompatibilities
Alkaline substances, acid, strong oxidizers, such as perchlorates, peroxides, chlorates, nitrates, permanganates.
Waste Disposal
Alkaline hydrolysis is the recommended mode of disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Precautions
During use/handling of carbofuran, workers should wear coveralls or a long-sleeved uniform, head covering, and chemical protective gloves made of materials such as rubber, neoprene, or nitrile. Occupational workers should know that areas treated with carbofuran are hazardous. The runoff of carbofuran material and the fi re control releases irritating or poisonous gases. It is advisable that workers should enter storehouses or carbofuran-treated close spaces with caution
Properties of Carbofuran
| Melting point: | 150-153 °C(lit.) |
| Boiling point: | 200°C |
| Density | 1.18 |
| vapor pressure | 2 x 10-5 mmHg at 33 °C (quoted, Verschueren, 1983) |
| refractive index | 1.5200 (estimate) |
| storage temp. | 0-6°C |
| solubility | Methylene chloride (>200 g/L), 2-propanol (20–50 g/L) (Worthing and Hance, 1991) |
| pka | 12.28±0.46(Predicted) |
| form | Powder |
| color | White, brown |
| Water Solubility | Slightly soluble. 0.07 g/100 mL |
| Merck | 13,1813 |
| BRN | 1428746 |
| Henry's Law Constant | 3.88 (x 10-8 atm?m3/mol)at 30 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | OSHA PEL: TWA 0.1 mg/m3; ACGIH TLV: TWA 0.1 mg/m3. |
| CAS DataBase Reference | 1563-66-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbofurane(1563-66-2) |
| EPA Substance Registry System | Carbofuran (1563-66-2) |
Safety information for Carbofuran
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. |
Computed Descriptors for Carbofuran
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
