Calcium sulfate dihydrate
Synonym(s):Calcium sulfate dihydrate
- CAS NO.:10101-41-4
- Empirical Formula: CaH6O5S
- Molecular Weight: 158.18
- MDL number: MFCD00149625
- EINECS: 231-900-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
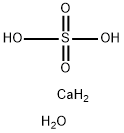
What is Calcium sulfate dihydrate ?
Chemical properties
monoclinic; hardness 1.5–2.0 Mohs; lumps or white powder(s); used in manufacturing portland cement, plaster of paris and artificial marble [KIR78] [MER06] [STR93]
The Uses of Calcium sulfate dihydrate
Calcium Sulfate Dihydrate is obtained from gypsum, a naturally occurring compound. Also used in the preparation of calcium sulfate and calcium sulfoaluminate.
The Uses of Calcium sulfate dihydrate
Used in this determination of oxalates
Definition
A mineral that consists of calcium sulfate with water molecules attached, or the rock that consists primarily of this mineral.
Definition
Gypsum: a monoclinic mineralform of hydrated calcium sulphate,CaSO4.2H2O. It occurs in five varieties:rock gypsum, which is oftenred stained and granular; gypsite, animpure earthy form occurring as asurface deposit; alabaster, a purefine-grained translucent form; satinspar, which is fibrous and silky; andselenite, which occurs as transparentcrystals in muds and clays. It is usedin the building industry and in themanufacture of cement, rubber,paper, and plaster of Paris.
Hazard
Nasal symptoms.
Agricultural Uses
Selenite, which is a salt of selenic acid (H2SeO3), and very similar to sulphite, is made by the oxidation of selenium by nitric acid. The low solubility of ironselenite complexes is responsible for the non-toxic level of selenium in plants growing on acid soils having a very high total selenium content. Plants absorb selenite, but generally to a lesser extent, than selenate, which is another form of selenium.
Safety Profile
Human systemic effects by inhalation: fibrosing alveolitis ('growth of fibrous tissue in the lung), unspecified respiratory system effects, and unspecified effects on the nose. Questionable carcinogen with experimental carcinogenic data. Long considered a nuisance dust (dependmg on silica content). When heated to decomposition it emits toxic fumes of SO,. See also CALCIUM SULFATE, CALCIUM COMPOUNDS, and SULFATES.
Synthesis
Calcium sulfate dehydrate (CSD) was firstly synthesized via the reaction of H2SO4 and Ca(OH)2 using ethanol as morphology modifier.
Purification Methods
It loses only part of its H2O at 100-150o (see below). It is soluble in H2O and very slowly soluble in glycerol. It is insoluble in most organic solvents.
Properties of Calcium sulfate dihydrate
| Melting point: | 128°C -1.5H₂O |
| Boiling point: | 163°C -2H₂O |
| Density | 2.32 |
| Flash point: | 163°C -2H₂O |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.01 M at 20 °C, clear, colorless |
| form | powder |
| color | White |
| Specific Gravity | 2.32 |
| PH | 7.0 (50g/l, H2O, 20℃)(slurry) |
| Odor | at 100.00?%. odorless |
| Water Solubility | 2 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1706 |
| Solubility Product Constant (Ksp) | pKsp: 4.5 |
| Dielectric constant | 2.5(Ambient) |
| Exposure limits | ACGIH: TWA 10 mg/m3 |
| Stability: | Stable. Incompatible with aluminium, strong acids. |
| CAS DataBase Reference | 10101-41-4(CAS DataBase Reference) |
| NIST Chemistry Reference | calcium sulfate(10101-41-4) |
| EPA Substance Registry System | Calcium sulfate dihydrate (10101-41-4) |
Safety information for Calcium sulfate dihydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Calcium sulfate dihydrate
| InChIKey | PASHVRUKOFIRIK-UHFFFAOYSA-L |
Calcium sulfate dihydrate manufacturer
JSK Chemicals
Oasis Fine Chem
Kronox Lab Sciences Pvt Ltd
HRV Global Life Sciences
Sujata Chemicals
Nandu Chemical Industries
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 10101-41-4 98%View Details
10101-41-4 98%View Details
10101-41-4 -
 Calcium-sulfate dihydrate 99%View Details
Calcium-sulfate dihydrate 99%View Details -
 Calcium sulfate dihydrate CAS 10101-41-4View Details
Calcium sulfate dihydrate CAS 10101-41-4View Details
10101-41-4 -
 Calcium sulfate dihydrate CAS 10101-41-4View Details
Calcium sulfate dihydrate CAS 10101-41-4View Details
10101-41-4 -
 Calcium sulfate, dihydrate CAS 10101-41-4View Details
Calcium sulfate, dihydrate CAS 10101-41-4View Details
10101-41-4 -
 Calcium-sulfate dihydrate 98%View Details
Calcium-sulfate dihydrate 98%View Details -
 Calcium Sulphate Dihydrate pure CAS 10101-41-4View Details
Calcium Sulphate Dihydrate pure CAS 10101-41-4View Details
10101-41-4 -
 Calcium sulfate dihydrate CAS 10101-41-4View Details
Calcium sulfate dihydrate CAS 10101-41-4View Details
10101-41-4