Barium sulfate
Synonym(s):Barium sulfate;Baryte;Blanc fixe
- CAS NO.:7727-43-7
- Empirical Formula: BaO4S
- Molecular Weight: 233.39
- MDL number: MFCD00003455
- EINECS: 231-784-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
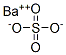
What is Barium sulfate?
Absorption
Barium sulfate is not absorbed following oral or rectal administration with a normal gastrointestinal tract. In patients with a normal GI tract, barium sulfate is normally excreted within 24 hr after oral ingestion. Post rectal administration of barium sulfate suspensions, the drug is generally excreted when the enema is released. Some barium may remain in the colon for several weeks, however, and eventually clears, especially in patients with impaired intestinal peristalsis . It is difficult to quantify the uptake of ingested barium because of a number of factors affect its absorption. The presence of sulfate in food can cause the precipitation of barium sulfate .
The following is the approximate time to peak opacification of organs by barium sulfate in a healthy GI tract:
Esophagus, stomach, and duodenum uptake of barium sulfate occurs almost immediately after oral administration .
Small intestine uptake is dependent on gastric emptying rate and viscosity of the preparation; it may be delayed 15-90 minutes post ingestion .
Small intestine (enteroclysis studies) uptake is immediate, following direct instillation .
Colon and distal small intestine uptake are dependent on patient positioning. Hydrostatic pressure also determines the rate and degree of opacification .
Toxicity
Acute Exposure
Nausea, vomiting, diarrhea and abdominal cramping may occur . Acute exposure to barium sulfate may irritate the eyes and respiratory tract. Exposure to inhalation or other forms can affect the nervous system and lead to hypokalemia, which can contribute to cardiovascular rhythm abnormality . Hypersensitivity, gastrointestinal transit delay, obstruction, aspiration pneumonitis and systemic embolization of barium sulfate are more serious complications of administration . In addition, fatalities have occurred due to aspiration pneumonitis, barium sulfate impaction, intestinal perforation with subsequent peritonitis and granuloma formation, and vasovagal and syncopal episodes , .
*Chronic Exposure *
The lungs may be affected by repeated or prolonged exposure to dust particles, resulting in baritosis (a type of benign pneumoconiosis) . Inhalation of barium sulfate dust may lead to a benign pneumoconiosis ("baritosis") with conspicuous radiographic characteristics but no signs of impairment of pulmonary function .
Intra-abdominal leakage
Intra-abdominal leakage may occur during or after administration of barium sulfate . Caution is advised in patients with a history of food aspiration and in patients with diagnosed swallowing disorders .
** A note on GI perforation**
Perforation of the colon after rectal administration of barium sulfate suspension has been reported due to the increased hydrostatic pressure of the instilled suspension, trauma to the colon from an enema tip, or forceful or deep insertion of a non-flexible enema tip. Perforation of the bowel has been followed by peritonitis, adhesions, granulomas, and death . This is a rare occurrence. Injury to the rectal mucosa or anal canal due to the enema tip or retention balloon is likely the most common traumatic cause of perforation during treatment. Inflation of a balloon within a stricture, neoplasm, inflamed rectum, or stoma is hazardous, and caution should be exerted .
Carcinogenicity and mutagenicity
No animal studies have been performed to evaluate the carcinogenicity of barium sulfate or potential effects on reproduction . Elective contrast radiography of the abdomen is not routintely recommended during pregnancy because of the risks to the fetus from radiation exposure .
Description
Barium sulfate is available as odourless, tasteless, white or yellowish crystals or powder or polymorphous crystals. It is stable and insoluble or negligibly soluble in water, and on burning, it may produce sulphur oxides. It reacts violently with aluminium powder. It occurs naturally as mineral barite, barytes. It has wide use as inert filler and pigment extender in paints, primers, inks, plastics, floor tiles, paper coatings, polymer fibres, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixotropic weighting mud in oil well drilling. Barium sulphate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the oesophagus, the stomach, and/ or the small intestine. The raw barium sulphate has wide applications such as in the manufacture of lithopone, a white pigment in the manufacture of photographic paper, wallpaper and glassmaking, in battery plate expanders, and in heavy concrete for radiation shield.
Description
Barium sulfate?(BaSO4), also known as barite, is a dense (4.48 g/cm3), insoluble salt that has many uses. Almost all barite (98%) is used as a densifying additive to oil well drilling fluids, or “muds”, which are used to seal the space around the drill bit. The mud also flushes out chippings and cools and lubricates the bit. High-density muds are necessary for drilling into deep formations to counteract high gas pressures. BaSO4?also has multiple uses in X-ray imaging, paint pigments, fireworks, and brake linings.
Chemical properties
Barium sulfate is available as white powder or polymorphous crystals. It is stable, odor- less, insoluble, or negligibly soluble in water, and may produce sulfur oxides on burning. It occurs naturally as mineral barite (barytes). It has wide use as an inert i ller pigment extender in paints, primers, inks, plastics, l oor tiles, paper coatings, polymer i bers, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixo- tropic weighting mud in oil well drilling. Barium sulfate is radio-opaque and is used as bar- ium meal in medical x-ray diagnosis. Barium sulfate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the esophagus, the stomach, and/or the small intestine. Barium sulfate is the raw material for the manufacture of litho- pone, a white pigment, and is used in the manufacture of photographic paper, wallpaper, and glassmaking; in battery plate expanders; and in heavy concrete for radiation shield.
Physical properties
Barium sulfate has the molecular formula of BaSO4
and the molecular weight of 233.3896 g/mol. It can be
prepared by the reaction of barium carbonate and
sulfuric acid:
BaCO3+H2SO4?BaSO4+CO2+H2O
Barium sulfate is a soft crystalline solid. It is
a rhombic crystal. The pure salt is white but the color
of the mineral “barite” can vary between red, yellow,
gray or green, depending on impurities. Its density is
4.50 g/cm3 and its refractive index is 1.64. It melts
around 1580°C but decomposes above 1600°C. Its
hardness is 4.3 to 4.6 Mohs. It is virtually insoluble in
water (285 mg/l at 30°C) and insoluble in alcohol. Its
Ksp is 1.1×10-10. It is soluble in concentrated sulfuric
acid. The crystal structure of BaSO4 is known to be rhombic, with a space group pnma. The lattice parameters are: a=8.896? , b=5.462°, c=7.171 ?,
V=348.4 ?3.
Silica is the prime impurity that can be removed as
sodium fluorosilicate by treatment with hydrofluoric
acid followed by caustic soda. Very pure barium sulfate
may be obtained by treating an aqueous solution of
a soluble barium salt with sodium sulfate:
BaCl2+Na2SO4?BaSO4+2NaCl
Barium sulfate is one of the most insoluble salts of the
alkaline earths. It does not undergo double decomposition
reactions in aqueous phase like its Mg homologue.
It dissolves in concentrated H2SO4 to form an acid
sulfate that breaks down to BaSO4 upon dilution. Reduction
with coke under heating produces barium sulfide:
BaSO4+3C?BaS+2CO+CO2
Occurrence
Barium sulfate is widely distributed in nature and occurs as the mineral barite (also known as barytes or heavy spar). It often is associated with other metallic ores, such as fluorspar. Barites containing over 94% BaSO4 can be processed economically.
Barium sulfate has many commercial applications. It is used as natural barite, or precipitated BaSO4. The precipitated salt in combination with equimolar amount of co-precipitated zinc sulfide formerly was used as a white protective coating pigment, known as lithophone. Similarly, in combination with sodium sulfide, it is used to produce fine pigment particles of uniform size, known as blanc fixe. Natural barite, however, has greater commercial application than the precipitated salt. It is used as drilling mud in oil drilling to lubricate and cool the drilling bit, and to plaster the walls of the drill hole to prevent caving. It is used as a filler in automotive paints, plastics and rubber products. It also is used in polyurethane foam floor mats; white sidewall rubber tires; and as a flux and additive to glass to increase the refractive index.
Other chemical applications of barium sulfate are as the opaque ingredient in a barium meal for x-ray diagnosis; as a pigment for photographic paper; and to prepare many barium salts.
The Uses of Barium sulfate
barium sulfate is an emulsion stabilizer for sunscreen formulations. outside of sunscreen preparations, this inorganic salt is most commonly used in non-cosmetic soaps.
The Uses of Barium sulfate
Finepowder Barium Sulphate is widely used in chemical industrypaint, plastics, rubber,glass, paper, medicine,ceramics, storage battery and other areas;Fine powder Barium Sulphate is widely used in chemical industry
The Uses of Barium sulfate
A heavy, white powder made by treating barium salts with sulfuric acid. Barium sulfate was used as a preliminary coating for raw photographic papers to produce a smooth, white surface and to act as a barrier to prevent reactions between the paper and subsequent coatings of gelatin or collodion emulsions. Barium sulfate was also added directly to emulsions to produce a matte finish. It is also known as barite, synthetic barite, blanc fix, baryta white, and mountain snow.
The Uses of Barium sulfate
Barium sulfate has many commercial applications. It
is used either as natural barite, or precipitated BaSO4.
The precipitated salt in combination with equimolar
amount of co-precipitated zinc sulfide formerly was
used as a white protective coating pigment, known as
“lithopone”. Similarly, in combination with sodium
sulfide, it is used to produce fine pigment particles of
uniform size, known as “blanc fixe”. Natural barite,
however, has greater commercial application than the
precipitated salt. It is used as an additive in drilling
mud in crude oil, well drilled to lubricate and cool the
drilling bit, and to plaster the walls of the drill hole to
prevent caving. It is used as a filler in automotive paints,
plastics and rubber products. It also is used as a filler in
polyurethane foam floor mats, white sidewall rubber
tires and as a flux and additive to glass to increase the
refractive index.
Barium sulfate is frequently used clinically as
a contrast agent for X-ray imaging and other diagnostic
procedures. It is most often used in imaging of the
gastrointestinal tract. It is administered, orally or by
enema, as a suspension of fine particles in an aqueous
solution. Although barium, and its water-soluble
compounds are often highly toxic, the extremely low
solubility of barium sulfate protects the patient from
absorbing harmful amounts of the metal. Barium sulfate
is also readily removed from the body, unlike prior
compounds, which it replaced. Its absorbance of Xrays
is also higher.
Barium sulfate mixtures are used as white pigment
for paints. In oil paint, barium sulfate is almost transparent,
and is used as a filler or to modify consistency.
One major manufacturer of artists’ oil paint sells
“permanent white” that contains a mixture of titanium
white pigment and barium sulfate. Barium
sulfate itself is called blanc fixe (French for “permanent
white”).
range. It is applied by spray painting to almost any
substrate (metals, plastics, glass) for use in integrating
spheres, laser cavities, lamp reflectors and display backlights.
It is characterized by a near-perfect Lambertian
(i.e. diffuse) reflectance of up to 98% in the spectral
range from 250 to 2500 nm.
Other chemical applications of barium sulfate are
used as a pigment for photographic paper. It is also
used to prepare many other barium salts. It is available
in many forms commercially.
Background
Barium sulfate is an inorganic compound with the chemical formula BaSO4 .
Barium sulfate occurs in nature as the mineral barite. It is also used in various manufacturing applications and mixed into heavy concrete to serve as a radiation shield .
This drug is used as a contrast agent in diagnostic x-ray procedures. Therapeutic advantages of barium sulfate in diagnostic procedures include both its low water solubility and high level of clearance from the body .
Barium sulfate is ingested by mouth or administered rectally and combined with granules of effervescent bicarbonate to enhance distension of the GI tract, allowing for enhanced gastrointestinal tract visualization , .
Indications
Barium sulfate is a radiographic contrast agent indicated for use in computed tomography (CT) of the abdomen to delineate the gastrointestinal (GI) tract in adult and pediatric patients .
Definition
ChEBI: A metal sulfate with formula BaO4S. Virtually insoluble in water at room temperature, it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite.
Production Methods
Natural barium sulfate or barite is widely distributed in nature. It also contains silica, ferric oxide and fluoride impurities. Silica is the prime impurity which may be removed as sodium fluorosilicate by treatment with hydrofluoric acid followed by caustic soda.
Very pure barium sulfate may be precipitated by treating an aqueous solution of a barium salt with sodium sulfate:
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively.
brand name
Baricon (Mallinckrodt); Bar-test (Glenwood); Barocat (Mallinckrodt); Barosperse (Mallinckrodt); Barosperse II (Mallinckrodt); Barotrast (Rhone-Poulenc Rorer); Epi-C (Mallinckrodt); Epi-Stat 57 (Mallinckrodt); Epi-Stat 61 (Mallinckrodt); Esophotrast (Rhone-Poulenc Rorer); Oratrast (Rhone-Poulenc Rorer).
General Description
Barium sulphate is widely employed as an inorganic filler. Its crystals belong to the rhombic crystal system. The rhombic crystals transforms to monoclinic form at 1148°C and eventually it decomposes to barium oxide, sulfur dioxide and oxygen at temperatures above 1400°C.
Reactivity Profile
Barium sulfate is non-combustible and non-toxic. Emits toxic sulfur oxides when heated to decomposition. Can act as an oxidizing agent, but usually does not. Reacts with reducing agents such as potassium, phosphorus or aluminum (heating with aluminum can cause an explosion).
Hazard
Pneumoconiosis.
Health Hazard
Exposures to barium sulfate cause irritation to the eyes, lachrimation; redness, scaling, and itching are characteristics of skin inl ammation. Although barium sulfate has been identi- i ed as a non-toxic dust, long-term inhalation of dust in high concentrations has caused benign pneumoconiosis (baritosis), deposition of dust in the lungs in sufi cient quantities to produce adverse effects. This produces a radiological picture even though symptoms and abnormal signs may not be present. The Fumes of barium are respiratory irritants and over-exposure to dusts and fumes is known to cause rhinitis, frontal headache, wheezing, laryngeal spasm, salivation, and anorexia. Long-term effects include nervous disorders and adverse effects on the heart, circulatory system, and musculature. Heavy exposures to barium sulfate may cause benign pneumoconi in exposed workers. However, there are no reports indicating that barium sulfate has potential occupational hazards or carcinogenicity.
Pharmaceutical Applications
Barium salts can be highly toxic even at low concentrations. Barium carbonate is highly toxic and can be used as rat poison as it readily dissolves in the stomach acid. Barium sulfate is the least toxic barium compound mainly because of its insolubility. Barium sulfate is used in a variety of applications ranging from white paint to X-ray contrast agent. The clinical use of barium sulfate suspension is well known under the term barium meal. Patients are given a suspension of barium sulfate to swallow. Using X-ray imaging, the whole oesophagus, the stomach and the intestines can be visualised. Barium sulfate lines the tissue whilst travelling through the digestive tract. The heavy barium ions absorb X-rays readily and therefore these structures become visible in an X-ray screening. Barium sulfate is a well-used and tolerated oral radio-contrast agent. It is also used as radio-contrast agent in enemas.
Pharmacokinetics
Barium sulfate increases the absorption of x-rays as they are passed throughout the body, delineating body structures, in which barium sulfate is localized. This allows for the clear visualization of normal organs/defect in normal anatomy .
Safety Profile
Questionable carcinogen with experimental tumorigenic data. Mutation data reported. A relatively insoluble salt used as an opaque medium in radtography. Soluble impurities can lead to toxic reactions. Heating with aluminum can produce an explosion. Incompatible with aluminum and potassium. When heated to decomposition it emits toxic fumes of SOx.
Potential Exposure
Barium sulfate is used as an opaque medium in radiography; as a mud weighting material in oil well drilling; in paper coating; as a paint pigment.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, rinsemouth and get medical attention immediately due to thepossibility of barium poisoning. See also “First Aid” sectionin “Barium” entry
Metabolism
Barium sulfate is poorly water soluble and shows negligible levels of absorption from the gastrointestinal tract following both oral or rectal administration. In healthy subjects, orally administered barium sulfate is generally excreted within 24 hours. Rectally administered barium sulfate is eliminated with clearance of the enema .
storage
Barium sulfate should be kept stored in its original sealed glass container with proper security when not in use. Barium sulfate should be stored in a cool, dry, ventilated area, away from incompatible materials and foodstuff containers. The containers need to be protected against physical damage and checked regularly for leaks.
Shipping
UN1564 Barium compounds, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Incompatibilities
May act as an oxidizer. Reacts with reducing agents such as hydrides, potassium, phosphorus or aluminum. Aluminum powder1heat may be violent; possibly explosive.
Precautions
Barium sulfate should only be used by or under the direct supervision of a qualii ed super- visor or doctor. During use and handling of barium sulfate, workers should avoid contact of the chemical substance with the skin and should not breathe dust. After use, the work- ers should wash their hands with soap and water. While dispensing, care is needed to label barium sulfate properly and correctly, to avoid confusion with poisonous barium suli de, suli te, or carbonate.
Properties of Barium sulfate
| Melting point: | 1580 °C |
| Boiling point: | decomposes at 1580℃ [KIR78] |
| Density | 4.5 |
| storage temp. | Storage temperature: no restrictions. |
| solubility | water: insoluble |
| form | powder |
| color | White to yellow |
| Specific Gravity | 4.5 |
| PH Range | 7 |
| PH | 3.5-10.0 (100g/l, H2O, 20℃)suspension |
| Odor | wh. or ylsh. fine powd. free from grittiness, odorless, tasteless |
| Water Solubility | 0.0022 g/L (50 ºC) |
| Merck | 14,994 |
| Solubility Product Constant (Ksp) | pKsp: 9.97 |
| Exposure limits | ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Dielectric constant | 11.4(16.0℃) |
| Stability: | Stable. |
| CAS DataBase Reference | 7727-43-7(CAS DataBase Reference) |
| EPA Substance Registry System | Barium sulfate (7727-43-7) |
Safety information for Barium sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Barium sulfate
Barium sulfate manufacturer
JSK Chemicals
Mumal Microns Pvt Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
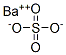
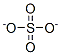
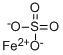
You may like
-
 BARYTES POWDER 99%View Details
BARYTES POWDER 99%View Details -
 Barium sulfate 99%View Details
Barium sulfate 99%View Details -
 Barium sulfate CAS 7727-43-7View Details
Barium sulfate CAS 7727-43-7View Details
7727-43-7 -
 Barium sulfate CAS 7727-43-7View Details
Barium sulfate CAS 7727-43-7View Details
7727-43-7 -
 Barium sulfate CAS 7727-43-7View Details
Barium sulfate CAS 7727-43-7View Details
7727-43-7 -
 Barium sulfate 98%View Details
Barium sulfate 98%View Details -
 Barium sulfate CAS 7727-43-7View Details
Barium sulfate CAS 7727-43-7View Details
7727-43-7 -
 Barium sulfate CAS 7727-43-7View Details
Barium sulfate CAS 7727-43-7View Details
7727-43-7
