calcium sulfate
Synonym(s):Calcium sulfate dihydrate;Gypsum
- CAS NO.:99400-01-8
- Empirical Formula: CaO4S
- Molecular Weight: 136.14
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
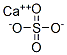
What is calcium sulfate?
The Uses of calcium sulfate
Calcium Sulfate is a general additive available as both calcium sul- fate anhydrous, made by the high-temperature calcining of gypsum which is then ground and separated, and calcium sulfate dihydrate, which is made by grinding and separating gypsum containing about 20% water of crystallization. calcium sulfate anhydrous contains approximately 29% calcium, and calcium sulfate dihydrate contains approximately 23% calcium. it is used, among other things, as a filler and baking powder for standardization purposes; a firming agent in canned potatoes, tomatoes, carrots, lima beans, and pep- pers; in dough as a source of calcium ions (because the absence of calcium ions causes bread dough to be soft and sticky and to pro- duce bread of poor quality); in soft-serve ice cream to produce dry- ness and stiffness; as a calcium ion source for reaction with alginates to form dessert gels; and as a calcium source for food enrichment.
The Uses of calcium sulfate
Pharmaceutic aid (tablet and capsule diluent).
The Uses of calcium sulfate
Calcium Sulfate is most often used as “gypsum” which is the dihydrate. In the form of b-anhydrite (the nearly anhydrous form), it is used as a desiccant. It is also used as a coagulant in products like “tofu”, which is a “bean curd”. When sold as a colorindicating variant under the name “Drierite ”, it appears blue or pink due to impregnation with cobalt chloride, which functions as a moisture indicator. In its natural state, unrefined calcium sulfate is a translucent, crystalline white rock. Many forms are known, including “Alabaster” and “Gypsum”. Selenite, Satin spar, Desert rose, and Gypsum flower are four varieties of Gypsum minerals; all four varieties are crystalline. The four “crystalline” varieties of gypsum are sometimes grouped together and called selenite. All have the same chemical formulation, CaSO4·2H2O. All varieties of gypsum are very soft minerals (hardness: 2 on Mohs scale). This is the most important identifying characteristic of gypsum.
Preparation
Calcium Sulfate may be
prepared by the addition of a soluble calcium salt to
a solution of sodium sulfate:
CaCl2+ Na2SO4→CaSO4+ 2NaCl
The product is generally a dihydrate although other
hydrates are also known. The CAS numbers of these
materials are: 7778-18-9 (anhydrite); 10034-76-1 (hemihydrate); 10101-41-4 (dihydrate).
Definition
ChEBI: Calcium sulfate is a calcium salt and an inorganic calcium salt.
Industrial uses
Calcium sulfate (CaSO4?2H2O) is the common blackboard chalk.
Properties of calcium sulfate
| Melting point: | >300°C |
| storage temp. | Hygroscopic, Room Temperature, under inert atmosphere |
| solubility | Water (Slightly) |
| form | Solid |
| color | Off-White to Pale Grey |
| Solubility Product Constant (Ksp) | pKsp: 4.31 |
| Dielectric constant | 5.6(0.0℃) |
Safety information for calcium sulfate
Computed Descriptors for calcium sulfate
calcium sulfate manufacturer
SGS & Company
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
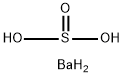
You may like
-
 Calcium sulfate 99%View Details
Calcium sulfate 99%View Details -
 Calcium sulfate 98%View Details
Calcium sulfate 98%View Details -
 Gypsum CAS 99400-01-8View Details
Gypsum CAS 99400-01-8View Details
99400-01-8 -
 Calcium Sulfate CAS: 7778-18-9View Details
Calcium Sulfate CAS: 7778-18-9View Details
7778-18-9 -
 Powder 500Kg Calcium Sulfate, Technical Grade, Packaging Size: 50 kgView Details
Powder 500Kg Calcium Sulfate, Technical Grade, Packaging Size: 50 kgView Details
7778-18-9 -
 Calcium SulfateView Details
Calcium SulfateView Details
99400-01-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
