Choline chloride
Synonym(s):(2-Hydroxyethyl)trimethylammonium chloride
- CAS NO.:67-48-1
- Empirical Formula: C5H14ClNO
- Molecular Weight: 139.62
- MDL number: MFCD00011721
- EINECS: 200-655-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
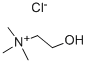
What is Choline chloride?
Chemical properties
White crystalline powder
The Uses of Choline chloride
Choline chloride helps in providing nourishment for pig feed and poultry feed. It acts as a food flavor enhancer. It is used as a precursor of acetylcholine and is a methyl donor in various metabolic processes and in lipid metabolism.
The Uses of Choline chloride
choleretic, lipotropic, hepatoprotectant
What are the applications of Application
Choline chloride has been used:
as a component of choline acetyltransferase (ChAT) buffer and for choline uptake in cultured human neuroblastoma (SK-N-SH) cells
for choline standard curve generation for quantifying phospholipase D (PLD) activity
as a component of slice solution for the dissection of entorhinal-hippocampal brain slices
Definition
ChEBI: Choline chloride is a quaternary ammonium salt with choline cation and chloride anion. It has a role as an animal growth promotant. It is a chloride salt and a quaternary ammonium salt. It contains a choline.
Preparation
Choline chloride can be made by treating trimethylamine with 2-chloroethanol.
(CH3)3N + ClCH2CH2OH → [(CH3)3NCH2CH2OH]+Cl?
General Description
Choline chloride appears as white crystals. Practically neutral aqueous solution(NTP, 1992). It is a basic constituent of lecithin that is found in many plants and animal organs. It is important as a precursor of acetylcholine, as a methyl donor in various metabolic processes, and in lipid metabolism.
Air & Water Reactions
Deliquescent. Very soluble in water.
Reactivity Profile
An acidic organic salt. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Fire Hazard
Choline chloride is relatively nonflammable.
Biochem/physiol Actions
The enzymatic activities of butyrylcholinesterase (BChE) and paraoxonase 1 (PON1), two serum enzymes synthesized by the liver and related with inflammation, were decreased in a sepsis animal model injected with LPS. Choline chloride administered intravenously at 20 mg/kg body weight prevents the LPS-mediated decreases in the activities of these two enzymes .
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic experimentally by ingestion and subcutaneous routes. Mutation data reported. A lipotropic agent which induces the reduction in fats contained in the liver. When heated to decomposition it emits toxic fumes of Cl-, SOx and NOx. See also CHOLINE.
Purification Methods
Extremely deliquescent. Check purity by AgNO3 titration or by titration of OH-base after passage through an anion-exchange column. Crystallise it from absolute EtOH, or EtOH/Et2O, dry it under vacuum and store it in a vacuum desiccator over P2O5 or Mg(ClO4)2. [Beilstein 4 IV 1443.]
Properties of Choline chloride
| Melting point: | 302-305 °C (dec.) (lit.) |
| Density | 1.0238 (rough estimate) |
| refractive index | 1.5400 (estimate) |
| FEMA | 4500 | Choline chloride (Also Includes Choline) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 1 M, clear, colorless |
| form | Adhering Crystals |
| color | White |
| PH | 5.0-7.5 (25℃, 1M in H2O) |
| Odor | sl. odor of trimethylamine |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.010 |
| Merck | 14,2206 |
| JECFA Number | 2003 |
| BRN | 3563126 |
| Stability: | Stable. Incompatible with strong oxidizing agents, moisture. Store under a dry atmosphere. |
| CAS DataBase Reference | 67-48-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Choline chloride(67-48-1) |
| EPA Substance Registry System | Choline chloride (67-48-1) |
Safety information for Choline chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Choline chloride
| InChIKey | SGMZJAMFUVOLNK-UHFFFAOYSA-M |
Choline chloride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Choline Chloride Pure 99%View Details
Choline Chloride Pure 99%View Details -
 CHOLINE CHLORIDE 75% 98%View Details
CHOLINE CHLORIDE 75% 98%View Details
67-48-1 -
 Choline Chloride 99%View Details
Choline Chloride 99%View Details -
 Choline chloride, 99% CAS 67-48-1View Details
Choline chloride, 99% CAS 67-48-1View Details
67-48-1 -
 Choline Chloride CAS 67-48-1View Details
Choline Chloride CAS 67-48-1View Details
67-48-1 -
 Choline chloride 97% CAS 67-48-1View Details
Choline chloride 97% CAS 67-48-1View Details
67-48-1 -
 Choline chloride CAS 67-48-1View Details
Choline chloride CAS 67-48-1View Details
67-48-1 -
 CHOLINE CHLORIDE Extra Pure CAS 67-48-1View Details
CHOLINE CHLORIDE Extra Pure CAS 67-48-1View Details
67-48-1
