Chromium(III) chloride
Synonym(s):Chromic chloride;Chromium trichloride
- CAS NO.:10025-73-7
- Empirical Formula: Cl3Cr
- Molecular Weight: 158.36
- MDL number: MFCD00010948
- EINECS: 233-038-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
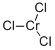
What is Chromium(III) chloride?
Absorption
Chromium absorption can increase with exercise.
Toxicity
Reported toxic reactions to chromium include nausea, vomiting, ulcers of the gastrointestinal tract, renal and hepatic damage, convulsions and coma. The acute LD50 for intravenous trivalent chromium in rats was reported as 10 to 18 mg/kg.
Description
Chromic chloride is a highly corrosive, blueor greenish to black crystalline solid. Molecularweight =158.35; Boiling point (decomposes) =1300℃;Freezing/Melting point =1152℃ (dissociates above1300℃). Hazard Identification (based on NFPA-704 MRating System): Health 3, Flammability 0, Reactivity 0.Insoluble in water (reaction)
Chemical properties
Chromic chloride is a highly corrosive, Blue or greenish to black crystalline solid.
The Uses of Chromium(III) chloride
Supplement(trace mineral).
Chromium(III) chloride and chromium(III) sulfate have been used as dietary supplements (approved for themanufacture of foods for particular nutritional uses and in food supplements in the European Union), whereas the organic chromium(III) complexes chromium picolinate and nicotinate are not approved in the European Union but find widespread uses (e.g. in the USA) inmultivitamin, multimineral products (Riihimaki & Luotamo, 2006).They are also marketed for weight loss purposes and athletic supplements.
The Uses of Chromium(III) chloride
Chromium chloride and chromium sulfate have been used as dietary supplements. Chromium(III) chloride and chromium(III) sulfate can be used in the manufacture of food for particular nutritional uses and in food supplements. It is found in miscellaneous applications, such as catalysts, hardeners, mordents, crosslinking and binding agents. It is used to make very pure chromium and other chromium compounds, as a mordant in dyeing cotton, and in chromizing metals. It is too used for trivalent electroplating .
Indications
For use as a supplement to intravenous solutions given for total parenteral nutrition (TPN).
Background
Chromic chloride, for injection, is a sterile, nonpyrogenic solution intended for use as an additive to solutions for Total Parenteral Nutrition (TPN).
What are the applications of Application
Chromium(III) chloride is a reagent for vapor-phase co-reductions with other metal halides
Air & Water Reactions
The rate of solution for water is extremely slow, pH of 0.2 M. aqueous solution is 2.4.
Reactivity Profile
When heated to decomposition, CHROMIUM (III) CHLORIDE emits toxic fumes of chlorine containing compounds. Violent reaction with lithium, nitrogen. Incompatible with strong oxidizers. [EPA, 1998].
Hazard
A poison.
Health Hazard
CHROMIUM (III) CHLORIDE displays high dermal toxicity, and moderate oral toxicity.
Flammability and Explosibility
Non flammable
Pharmacokinetics
Trivalent chromium is part of glucose tolerance factor, an essential activator of insulin-mediated reactions. Chromium helps to maintain normal glucose metabolism and peripheral nerve function. Providing chromium during TPN helps prevent deficiency symptoms including impaired glucose tolerance, ataxia, peripheral neuropathy and a confusional state similar to mild/moderate hepatic encephalopathy.
Safety Profile
Poison by skin contact, inhalation, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Human mutation data reported. Questionable carcinogen. Reacts violently with lithium under nitrogen atmosphere. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
Chromic chloride is used for production of solutions of basic chlorides and as a catalyst for polymerization of olefins and other organic reactions; in chromizing; in the manufacture of chromium metal and compounds; as a textile mordant; in tanning; in corrosion inhibitors; and as a waterproofing agent. A nutritional supplement.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Metabolism
The metabolism of chromic chloride hexahydrate in guinea pigs for 60 days after intratracheal injection of 200 ug Cr 10 min after injection, 69% of the dose remained in the lungs, & only 4% was found in the blood & liver, kidneys, spleen. By 24 hr, 45% was still in the lungs, 6% was excreted in the urine, & only a very small % was found in the other tissues. The spleen was the only tissue that showed accumulation & that occurred during the 1st 48 hr.
Storage
: Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. ColorCode—Green: General storage may be used. Prior to working with this chemical you should be trained on its properhandling and storage. Store in tightly closed containers in acool, well-ventilated area away from oxidizing agents andwater
Shipping
UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN1760 Corrosive liquids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Purification Methods
Sublime the chloride in a stream of dry HCl. Alternatively, the impure chromic chloride (100g) is added to 1L of 10% aqueous K2Cr2O7 and several millilitres of conc HCl, and the mixture is brought to a gentle boil with constant stirring for 10minutes. (This removed a reducing impurity.) The solid is separated and washed by boiling with successive 1L lots of distilled water until the wash water no longer gives a test for chloride ion, then dry it at 110o [Poulsen & Garner J Am Chem Soc 81 2615 1959, Hein & Herzog in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1338 1965].
Incompatibilities
Reacts with water and strong oxidizers. Contact with strong acids or acid fumes may produce highly toxic chloride fumes. May attack certain steels, causing pitting attack and stress corrosion
Waste Disposal
Precipitate as chromium hydroxide. Dewater the sludge and dispose of the compacted sludge in single-purpose dumps
Properties of Chromium(III) chloride
| Melting point: | 1152 °C |
| Boiling point: | 1300°C |
| Density | 2.87 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| storage temp. | Store below +30°C. |
| solubility | slightly soluble in H2O |
| form | flakes |
| color | Violet |
| Specific Gravity | 2.87 |
| Water Solubility | Soluble in alcohol, water. |
| Sensitive | Hygroscopic |
| Merck | 14,2222 |
| Exposure limits | NIOSH: IDLH 25 mg/m3; TWA 0.5 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 10025-73-7(CAS DataBase Reference) |
| EPA Substance Registry System | Chromium(III) chloride (10025-73-7) |
Safety information for Chromium(III) chloride
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Chromium(III) chloride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 10025-73-7 Chromium(III) chloride 99%View Details
10025-73-7 Chromium(III) chloride 99%View Details
10025-73-7 -
 Chromium(III) chloride CAS 10025-73-7View Details
Chromium(III) chloride CAS 10025-73-7View Details
10025-73-7 -
 Chromium(III) chloride CAS 10025-73-7View Details
Chromium(III) chloride CAS 10025-73-7View Details
10025-73-7 -
 Chromium(III) chloride CAS 10025-73-7View Details
Chromium(III) chloride CAS 10025-73-7View Details
10025-73-7 -
 Chromium(III) chloride, Anhydrous CAS 10025-73-7View Details
Chromium(III) chloride, Anhydrous CAS 10025-73-7View Details
10025-73-7 -
 Chromium(III) chloride, Anhydrous CAS 10025-73-7View Details
Chromium(III) chloride, Anhydrous CAS 10025-73-7View Details
10025-73-7 -
 Chromium(III) chloride CAS 10025-73-7View Details
Chromium(III) chloride CAS 10025-73-7View Details
10025-73-7 -
 CHROMIUM (III) CHLORIDE CAS 10025-73-7View Details
CHROMIUM (III) CHLORIDE CAS 10025-73-7View Details
10025-73-7
