Bismuth trichloride
Synonym(s):Bismuth trichloride;Bismuth(III) chloride;Trichlorobismuth
- CAS NO.:7787-60-2
- Empirical Formula: BiCl3
- Molecular Weight: 315.34
- MDL number: MFCD00003461
- EINECS: 232-123-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-18 19:10:02
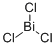
What is Bismuth trichloride ?
Chemical properties
Bismuth trichloride is white crystal and crystalline powder
Physical properties
Yellowish-white crystalline solid; cubic crystals; hygroscopic; density 4.75g/cm3; melts at 230°C; vaporizes at 447°C; vapor pressure 5 torr at 242°C; reacts with water; soluble in acids, alcohol and acetone.
The Uses of Bismuth trichloride
Bismuth trichloride used as catalyst for organic reactions.
The Uses of Bismuth trichloride
In the manufacture of other bismuth salts; as catalyst for organic reactions.
The Uses of Bismuth trichloride
Bismuth chloride (Bi3+ + 3Cl1- → BiCl3) reacts with water to produce what is known as “bismuth white” pigment.
Definition
Bismuth trichloride is a white deliquescent solid. It can be prepared by direct combination of bismuth and chlorine. Bismuth(III) chloride dissolves in excess dilute hydrochloric acid to form a clear liquid, but if diluted it produces a white precipitate of bismuth(III) chloride oxide (bismuthyl chloride, BiOCl): BiCl3 + H2O = BiOCl + 2HCl This reaction is often used as an exam ple of a reversible reaction and as a confirmatory test for bismuth during quantitative analysis. Bismuth(V) chloride does not form.
Preparation
Bismuth chloride may be synthesized directly by passing chlorine over bismuth. Alternatively, the chloride salt may be prepared by adding hydrochloric acid to basic bismuth chloride and evaporating the solution:
Bi(OH)2Cl + 2HCl → BiCl3 + 2H2O
Also, the compound can be prepared by dissolving bismuth in concentrated nitric acid and then adding solid sodium chloride into this solution. Another method of preparation is treating the metal with concentrated hydrochloric acid:
2Bi + 6HCl → 2BiCl3 + 3H2.
General Description
Bismuth(III) chloride is an inorganic salt that can be prepared by the reaction of bismuth with chlorine.
Purification Methods
Sublime the trichloride under high vacuum, or dry it under a current of HCl gas, followed by fractional distillation, once under HCl and once under argon. It is deliquescent. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 621 1963.]
Properties of Bismuth trichloride
| Melting point: | 230-232 °C(lit.) |
| Boiling point: | 447 °C(lit.) |
| Density | 4.75 |
| Flash point: | 430°C |
| storage temp. | no restrictions. |
| solubility | Soluble in hydrochloric acid, nitric acid, diethyl ether, ethyl acetate and acetone. Insoluble in alcohol. |
| form | powder |
| color | White to beige or gray |
| Specific Gravity | 4.75 |
| Odor | Hydrochloric acid odor |
| Water Solubility | decomposes |
| Sensitive | Hygroscopic |
| Sublimation | 430 ºC |
| Merck | 14,1262 |
| Stability: | Stable, but reacts with water, moisture. |
| CAS DataBase Reference | 7787-60-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Bismuth trichloride(7787-60-2) |
| EPA Substance Registry System | Bismuthine, trichloro- (7787-60-2) |
Safety information for Bismuth trichloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Bismuth trichloride
| InChIKey | JHXKRIRFYBPWGE-UHFFFAOYSA-K |
Bismuth trichloride manufacturer
JSK Chemicals
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 BISMUTH TRI CHLORIDE 99%View Details
BISMUTH TRI CHLORIDE 99%View Details -
 Bismuth(III) chloride 98%View Details
Bismuth(III) chloride 98%View Details -
 Bismuth(III) chloride, Ultra dry CAS 7787-60-2View Details
Bismuth(III) chloride, Ultra dry CAS 7787-60-2View Details
7787-60-2 -
 Bismuth(III) chloride, Ultra dry CAS 7787-60-2View Details
Bismuth(III) chloride, Ultra dry CAS 7787-60-2View Details
7787-60-2 -
 Bismuth(III) chloride CAS 7787-60-2View Details
Bismuth(III) chloride CAS 7787-60-2View Details
7787-60-2 -
 Bismuth(III) chloride CAS 7787-60-2View Details
Bismuth(III) chloride CAS 7787-60-2View Details
7787-60-2 -
 Bismuth Chloride (TRI)View Details
Bismuth Chloride (TRI)View Details
7787-60-2 -
 JCSSUPER 7787-60-2 Bismuth Chloride Extrapure (Bismuth Trichloride) 100 Gm.View Details
JCSSUPER 7787-60-2 Bismuth Chloride Extrapure (Bismuth Trichloride) 100 Gm.View Details
7787-60-2
