Vanadium(III) chloride
Synonym(s):Vanadium trichloride;Vanadium(III) chloride
- CAS NO.:7718-98-1
- Empirical Formula: Cl3V
- Molecular Weight: 157.29
- MDL number: MFCD00011454
- EINECS: 231-744-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
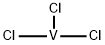
What is Vanadium(III) chloride?
Chemical properties
Vanadium(III) chloride is pink, deliquescent crystals that decompose upon heating. It is soluble in absolute alcohol and ether but decomposes in water. The hydrated ions of trivalent vanadium are green. They form hydrated coordinated chlorides with excess chloride ions and react with liquid ammonia to produce ammine compounds. They also react with gaseous ammonia to form nitrides, with amines and other organic compounds to produce corresponding coordination compounds, and with certain aromatic hydroxy acids to exhibit characteristic color reactions.
The Uses of Vanadium(III) chloride
Vanadium(III) chloride has been used as a catalyst for Biginelli condensation. As a reducing agent, it is capable of converting nitrate to nitrite in water samples, allowing for the direct detection of nitrates.
The Uses of Vanadium(III) chloride
Preparation of vanadium dichloride and organovanadium compounds.
What are the applications of Application
Vanadium(III) chloride is a common precursor to other vanadium(III) complexes
General Description
Vanadium(III) chloride is a crystalline solid with variable oxidation states. It is widely used as a catalyst in organic synthesis, polymerization processes, and redox batteries. It acts as a reducing agent.
Air & Water Reactions
Deliquescent. Generates acid mists when exposed to moist air. Dissolves in water with formation of an acidic solution and generation of acidic fumes.
Reactivity Profile
VANADIUM(III) CHLORIDE attacks many organic compounds. Can catalyze organic reactions. Aqueous solutions react as acids to neutralize bases. These neutralizations generate heat, but less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. Combination of the trichloride with methylmagnesium iodide, or other Grignard type reagents, can be violently explosive under a variety of conditions, Chem. Rev., 1955, 55, 560.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion and subcutaneous routes. A corrosive irritant to skin, eyes, and mucous membranes. Extremely violent reaction with methyl magnesium iodide and other Grignard reagents. When heated to decomposition it emits toxic fumes of VOx and Cl-. See also VANADIUM COMPOUNDS and HYDROCHLORIC ACID.
References
[1] BERNHARD SCHNETGER Carola L. Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent[J]. Marine Chemistry, 2014, 160: Pages 91-98. DOI:10.1016/j.marchem.2014.01.010.
[2] XU Toshikazu H. Vanadium-Catalyzed Pinacol Coupling Reaction in Water[J]. The Journal of Organic Chemistry, 2005, 70 21: 8594-8596. DOI:10.1021/jo051213f.
[3] GOWRAVARAM SABITHA. Vanadium(III) chloride: A mild and efficient catalyst for the chemoselective deprotection of acetonides[J]. Journal of Molecular Catalysis A: Chemical, 2005, 238 1: Pages 229-232. DOI:10.1016/j.molcata.2005.05.028.
[4] SHU WANG. Automated determination of nitrate plus nitrite in aqueous samples with flow injection analysis using vanadium (III) chloride as reductant.[J]. Talanta, 2016, 47 1: 744-748. DOI:10.1016/j.talanta.2015.06.031.
Properties of Vanadium(III) chloride
| Melting point: | 250 °C |
| Density | 3 g/mL at 25 °C(lit.) |
| storage temp. | no restrictions. |
| solubility | Soluble in tetrahydrofuran, alcohol, ether and hydrocarbons. |
| form | Powder |
| Specific Gravity | 3. |
| color | Dark blue to violet to black |
| Water Solubility | decomposes in H2O; soluble alcohol, ether [HAW93] |
| Sensitive | Moisture Sensitive |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| CAS DataBase Reference | 7718-98-1(CAS DataBase Reference) |
| EPA Substance Registry System | Vanadium chloride (VCl3) (7718-98-1) |
Safety information for Vanadium(III) chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Vanadium(III) chloride
Related products of tetrahydrofuran

You may like
-
 Vanadium(III) chloride CAS 7718-98-1View Details
Vanadium(III) chloride CAS 7718-98-1View Details
7718-98-1 -
 Vanadium(III) chloride CAS 7718-98-1View Details
Vanadium(III) chloride CAS 7718-98-1View Details
7718-98-1 -
 Vanadium(III) chloride, 97% CAS 7718-98-1View Details
Vanadium(III) chloride, 97% CAS 7718-98-1View Details
7718-98-1 -
 Vanadium(iii) chloride 95% CAS 7718-98-1View Details
Vanadium(iii) chloride 95% CAS 7718-98-1View Details
7718-98-1 -
 Vanadium(III) chloride CAS 7718-98-1View Details
Vanadium(III) chloride CAS 7718-98-1View Details
7718-98-1 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8