Blonanserin
- CAS NO.:132810-10-7
- Empirical Formula: C23H30FN3
- Molecular Weight: 367.5
- MDL number: MFCD00893838
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34

What is Blonanserin?
Description
Blonanserin, a dual antagonist of dopamine D2 and serotonin 5-HT2 receptors, was launched past year in Japan for the oral treatment of psychosis and schizophrenia. It is the latest entry in the class of atypical antipsychotic agents to reach the market. Blonanserin exhibits high affinity for D2 and 5-HT2 receptors (IC50 23.6 and 9.85 nM, respectively). Its affinity for D2 receptors is very close to that of haloperidol and risperidone, whereas the affinity for 5-HT2 receptors is about 7 times higher than that of haloperidol and 10 times lower than that of risperidone.Blonanserin is chemically derived in three steps starting with a polyphosphoric acid mediated condensation reaction of cyclooctanone with 4-fluorobenzoylacetonitrile. The resultant 4-fluorophenylcycloocta[b]pyridin-2-one intermediate is converted to the corresponding 2-chloro derivative by treatment with phosphoryl chloride and subsequently condensed with 1-ethylpiperazine to afford blonanserin.
Chemical properties
Pale Yellow Solid
Originator
Sumitomo (Japan)
The Uses of Blonanserin
Blonanserin is a novel atypical antipsychotic agent with potent dopamine D2 (Ki, 14.8 nM) and serotonin 5-HT2(Ki, 3.98 nM) receptors antagonist properties.
The Uses of Blonanserin
A 5-HT2 Serotonin receptor and D2 Dopamine receptor antagonist, used as an antipsychotic.
Definition
ChEBI: Blonanserin is an organic molecular entity.
Manufacturing Process
Preparation of 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-
hexahydrocyclooc ta[b]pyridine:
A mixture of 2-chloro-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta
[b]pyridine (2.0 g), N-ethylpiperazine (2.4 g), and potassium iodide (1.1 g) is
stirred at 170°C for 5 hours. After cooling, the reaction mixture is dissolved in
ethyl acetate and water. The organic layer is washed with water and extracted
with 5% hydrochloric acid. The extract is made alkaline with potassium
carbonate, and extracted with ethyl acetate. The extract is washed with water,
dried over anhydrous sodium sulfate, and concentrated under reduced
pressure.
(a) The residue is recrystallized from acetonitrile to give the desired product
(1.2 g), MP: 123°-124°C.
This product obtained in the above (a) is converted to the following salt
thereof by treating the product with various acids.
brand name
Lonasen
Therapeutic Function
Antipsychotic
Synthesis
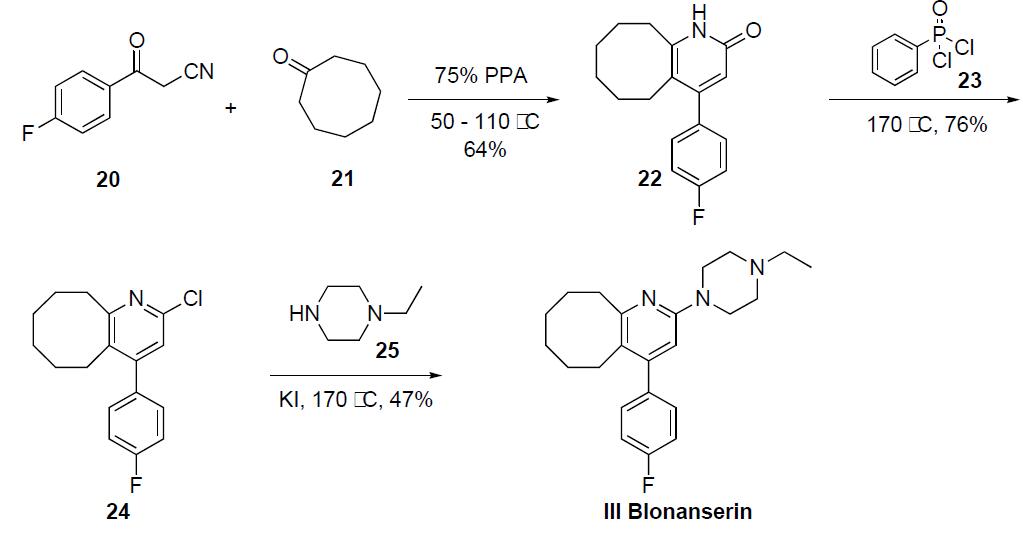
The synthesis of blonanserin III has been described in both the primary and patent literature in the following scheme. Condensation of cyclooctanone (21) with 4-fluorobenzoyl acetonitrile (20) in 75% polyphosphoric acid at 110 ??C provided the fused cyclooctapyridone 22 in 64% yield. Reaction of 22 with phenyldichlorophosphinic acid (23) at 170 ??C gave chloride 24 in 76% yield, which was then reacted with potassium idodide and 4-ethyl piperidine (25) at 170 ??C to give blonanserin (III) in 47% yield after crystallization from acetonitrile.
Properties of Blonanserin
| Melting point: | 117-119°C |
| Boiling point: | 540.8±50.0 °C(Predicted) |
| Density | 1.095±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: ≥10mg/mL |
| form | powder |
| pka | 7.66±0.10(Predicted) |
| color | white to off-white |
| Merck | 14,1319 |
Safety information for Blonanserin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Blonanserin
Blonanserin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Blonanserin 98%View Details
Blonanserin 98%View Details -
 132810-10-7 Blonanserin 99%View Details
132810-10-7 Blonanserin 99%View Details
132810-10-7 -
 132810-10-7 98%View Details
132810-10-7 98%View Details
132810-10-7 -
 Blonanserin CAS 132810-10-7View Details
Blonanserin CAS 132810-10-7View Details
132810-10-7 -
 Blonanserin CAS 132810-10-7View Details
Blonanserin CAS 132810-10-7View Details
132810-10-7 -
 Elicia Blonanserin 8 Tablet LonasenView Details
Elicia Blonanserin 8 Tablet LonasenView Details
132810-10-7 -
 Elicia Blonanserin 8 Tablet LonasenView Details
Elicia Blonanserin 8 Tablet LonasenView Details
132810-10-7 -
 Elicia Blonanserin 8mg TabletView Details
Elicia Blonanserin 8mg TabletView Details
132810-10-7
