ILOPERIDONE
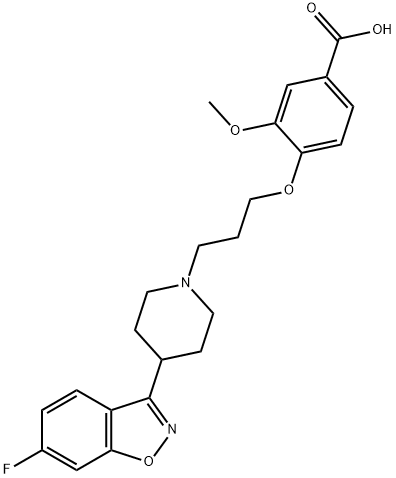
Synonym(s):1-[4-[3-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]ethanone;Iloperidone;Zomaril
- CAS NO.:133454-47-4
- Empirical Formula: C24H27FN2O4
- Molecular Weight: 426.48
- MDL number: MFCD00866688
- EINECS: 603-745-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
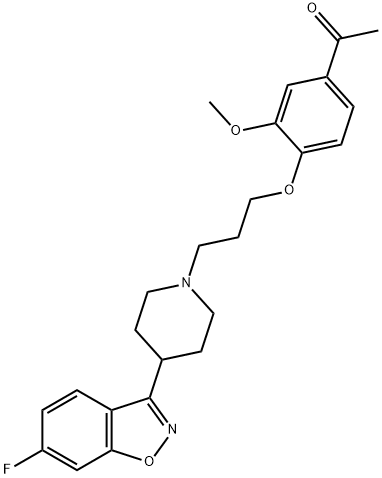
What is ILOPERIDONE?
Absorption
Well absorbed from the GI tract and Cmax is reached within 2-4 hours. Steady-state concentration is achieved in 3-4 days post-administration of iloperidone. Relative bioavailability of the tablet formulation compared to oral solution is 96%. Accumulation occurs in a predictable fashion.
Toxicity
Commonly observed adverse reactions (incidence ≥5% and two-fold greater than placebo) were: dizziness, dry mouth, fatigue, nasal congestion, orthostatic hypotension, somnolence, tachycardia, and weight increased.
Description
Iloperidone is an atypical antipsychotic and adrenergic, dopamine, and serotonin (5-HT) receptor antagonist. It binds to several receptors, including the α1-adrenergic receptor (α1-AR), α2-AR, and dopamine D2 receptor (Kis = 0.31, 3, and 3.3 nM, respectively), as well as the 5-HT1A, 5-HT1D, 5-HT2A, and 5-HT2C receptors (Kis = 33, 15, 0.2, and 14 nM, respectively) in radioligand binding assays using human post-mortem brain tissue. Iloperidone also binds to human D1, D3, D4, D5, and rat 5-HT2 receptors (Kis = 216, 7.1, 25, 319, and 3.1 nM, respectively, in CHO cells) and the histamine H1 receptor (Ki = 12.3 nM in human post-mortem brain tissue). Iloperidone (1-3 mg/kg) prevents the reduction in prepulse inhibition induced by apomorphine , phencyclidine (PCP), and cirazoline in rats. It also increases the time rats spend in the open arms of the elevated plus maze and the number of social interactions when administered at a dose of 0.5 mg/kg. Formulations containing iloperidone have been used in the treatment of schizophrenia.
Chemical properties
Off-White Solid
The Uses of ILOPERIDONE
Iloperidone (Fanapt, Fanapta, Zomaril) is an atypical antipsychotic for the treatment of schizophrenia. Iloperidone is a monoamine directed towards acting upon and antagonizing specific neurotransmitters, particularly multiple dopamine and serotonin recep
The Uses of ILOPERIDONE
Combined dopamine (D2) and serotonin (5HT2) receptor antagonist. Antipsychotic.
Indications
Treatment of acute schizophrenia.
Background
Iloperidone is an atypical antipsychotic for the treatment of schizophrenia symptoms. Hoechst Marion Roussel Inc. researched the drug until May 1996. In June 1997 they gave the research rights to Titan Pharmaceuticals, who gave the worldwide development, manufacturing, and marketing rights to Novartis in August 1998. On June 9, 2004, Titan Pharmaceuticals gave the Phase III development rights to Vanda Pharmaceuticals. FDA approved on May 9, 2009.
What are the applications of Application
Iloperidone is a dopamine (D2) and serotonin (5HT2) receptor antagonist
Definition
ChEBI: A member of the class of piperidines that is the 4-acetyl-2-methoxyphenyl ether of 3-(piperidin-1-yl)propan-1-ol which is substituted at position 4 of the piperidine ring by a 6-fluoro-1,2-benzoxazol-3-yl group. A member of the group of second generation a tipsychotics (also known as an atypical antipsychotics), it is used for the treatment of schizophrenia.
General Description
Iloperidone is an atypical antipsychotic administered for the treatment of schizophrenia. The drug is marketed under the trade names Fanapt and Fanapta. This Certified Spiking Solution? is suitable for use in LC/MS or GC/MS applications including clinical toxicology, forensic testing, or pharmaceutical research.
Biochem/physiol Actions
Iloperidone is an atypical antipsychotic, a combined dopamine (D2) and serotonin (5HT2) receptor antagonist. Iloperidone targets a selective set of dopamine, serotonin and also norepinephrine receptor subtypes. Like similar atypical antipsychotics, Iloperidone has higher affinity for the 5-HT2A receptor (Ki < 10 nM) than for the D2 receptor (Ki = 10–100) nM). Iloperidone has high affinity for dopamine D3 (Ki < 10 nM) and lower affinity for other serotonin or dopamine subtypes. Unlike most atypical antipsychotics, Iloperidone also has high affinity for norepinephrine alpha1 receptors (Ki < 10 nM) which may contribute to the drug′s efficacy on mood and cognition.
Pharmacokinetics
Iloperidone shows high affinity and maximal receptor occupancy for dopamine D2 receptors in the caudate nucleus and putamen of the brains of schizophrenic patients. The improvement in cognition is attributed to iloperidone's high affinity for α adrenergic receptors. Iloperidone also binds with high affinity to serotonin 5-HT2a and dopamine 3 receptors. Iloperidone binds with moderate affinity to dopamine D4, serotonin 5-HT6 and 5-HT7, and norepinephrine NEα1 receptors. Furthermore, iloperidone binds with weak affinity to serotonin 5-HT1A, dopamine D1, and histamine H1 receptors.
Clinical Use
Acting as an antagonist on serotonin (5-HT2) and dopamine receptor subtypes, iloperidone is an antipsychotic indicated for the treatment of acute schizophrenia in adults. Based on its in vitro and in vivo binding properties against both serotonin and dopamine receptors, it is expected that iloperidone will show fewer extrapyramidal symptoms than currently marketed antipsychotics such as haloperidol and clozapine. The original discovery was made by Hoechst-Roussel Pharmaceuticals who passed the developing rights to Vanda Pharmaceuticals and subsequently Novartis for marketing in the U.S. and Canada. While this drug was originally approved in the U.S. in 2009, the marketing was only initiated in the U.S. in 2010.
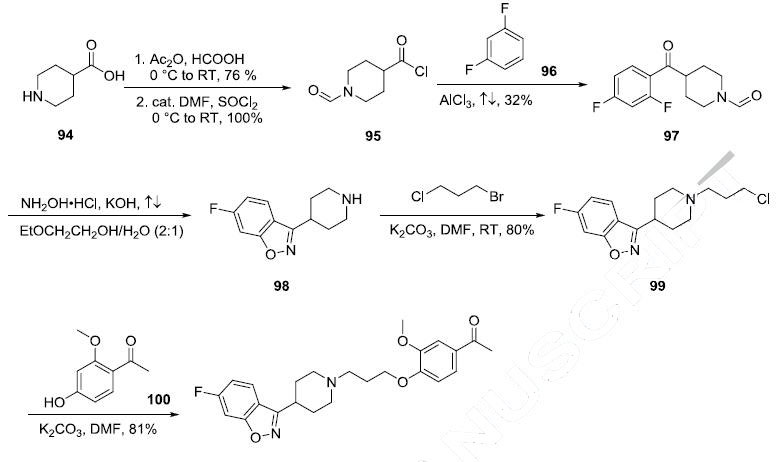
Synthesis
Although a number of syntheses are reported in the literature, the process enabled route from a key intermediate 98 is described in the scheme. The key intermediate 98 was synthesized from isonipecotic acid (94) in four steps. Formylation of isonipecotic acid (76% yield) followed by conversion of the acid to the acyl chloride gave 95 in 100% yield. Friedel-Crafts acylation of 1,3-difluorobenzene (96) with the acid chloride 95 provided ketone 97 in 32% yield. Treatment of ketone 97 with hydroxylamine hydrochloride in the presence of potassium hydroxide gave the corresponding oxime, which upon refluxing in 2-ethoxylethanol and water cyclized to piperidine benzisoxazole 98 with concomitant loss of the N-formyl group. Alkylation of piperidine 98 with 1-chloro-3-bromo propane in DMF in the presence of potassium carbonate provided the chloride intermediate 99 in 80% yield. Subsequent reaction with phenol 100 under basic conditions gave the desired product iloperidone (VII) in 81% yield.
Metabolism
Iloperidone is hepatically metabolized by cytochrome enzymes which mediates O-dealkylation (CYP3A4), hydroxylation (CYP2D6), and decarboxylation/reduction processes. Metabolites formed are P89, P95, and P88. The minor metabolite is P89, whereas P95 and P88 are the major ones. The affinity of the iloperidone metabolite P88 is generally equal or less than that of the parent compound. In contrast, the metabolite P95 only shows affinity for 5-HT2A (Ki value of 3.91) and the NEα1A, NEα1B, NEα1D, and NEα2C receptors (Ki values of 4.7, 2.7, 8.8 and 4.7 nM respectively).
Properties of ILOPERIDONE
| Melting point: | 118-120°C |
| Boiling point: | 593.7±50.0 °C(Predicted) |
| Density | 1.204±0.06 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear |
| form | powder |
| pka | 8.43±0.20(Predicted) |
| color | white to beige |
| Merck | 14,4900 |
Safety information for ILOPERIDONE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for ILOPERIDONE
ILOPERIDONE manufacturer
Megafine Pharma
Ralington Pharma
Symed Laboratories Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 133454-47-4 Iloperidone 98%View Details
133454-47-4 Iloperidone 98%View Details
133454-47-4 -
 133454-47-4 98%View Details
133454-47-4 98%View Details
133454-47-4 -
 133454-47-4 98%View Details
133454-47-4 98%View Details
133454-47-4 -
 133454-47-4 98%View Details
133454-47-4 98%View Details
133454-47-4 -
 133454-47-4 Iloperidone 98%View Details
133454-47-4 Iloperidone 98%View Details
133454-47-4 -
 Iloperidone 133454-47-4 99%View Details
Iloperidone 133454-47-4 99%View Details
133454-47-4 -
 Iloperidone CAS 133454-47-4View Details
Iloperidone CAS 133454-47-4View Details
133454-47-4 -
 Iloperidone CAS 133454-47-4View Details
Iloperidone CAS 133454-47-4View Details
133454-47-4