Zonisamide
Synonym(s):1,2-Benzisoxazole-3-methanesulfonamide;AD 810, CI 912;Zonisamide - CAS 68291-97-4 - Calbiochem
- CAS NO.:68291-97-4
- Empirical Formula: C8H8N2O3S
- Molecular Weight: 212.23
- MDL number: MFCD00865316
- EINECS: 614-395-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
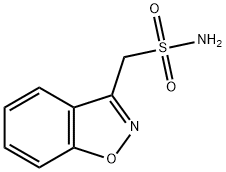
What is Zonisamide?
Absorption
Between 200 and 400 mg, zonisamide follows a dose-proportional pharmacokinetic profile. At concentrations higher than 800 mg, the Cmax and AUC increase in a disproportional manner, possibly due to zonisamide binding red blood cells. In healthy volunteers given 200 to 400 mg of zonisamide orally, peak plasma concentrations (Cmax) range between 2 and 5 μg/mL and are reached within 2–6 hours (Tmax). In healthy volunteers given 100 mg of zonisamide oral suspension, the Tmax ranged from 0.5 to 5 hours. Zonisamide has a high oral bioavailability (95%). The Tmax of zonisamide was delayed by food intake (4-6 hours); however, food has no effect on its bioavailability. Steady state is achieved 14 days after a stable dose is reached.
Toxicity
Information on daily doses over 800 mg/day of zonisamide is limited. During clinical development, three patients ingested unknown amounts of zonisamide as suicide attempts, and all of them were hospitalized with central nervous system symptoms. One patient became comatose and developed bradycardia, hypotension, and respiratory depression; 31 hours after zonisamide ingestion, plasma level was 100.1 μg/mL. Zonisamide plasma levels fell with a half-life of 57 hours, and the patient became alert five days later. There are no specific antidotes for zonisamide overdosage. In case of a suspected recent overdose, emesis should be induced or gastric lavage performed with the usual precautions to protect the airway. General supportive care is indicated, including frequent monitoring of vital signs and close observation. Due to its long half-life and low protein binding, renal dialysis may be effective in treating zonisamide overdose; however, the effectiveness of this procedure has not been formally studied.
In vivo studies found no evidence of carcinogenicity at zonisamide doses equivalent to or higher than the maximum recommended human dose (MRHD). In an in vitro chromosomal aberration assay in CHL cells, zonisamide displayed mutagenicity. Signs of reproductive toxicity were also detected in rats treated with a dose 0.5 times the MRHD.
Description
Zonisamide is a broad-spectrum antiepileptic effective in the treatment of refractory seizures. In cultured spinal cord neurons, zonisamide blocks the sustained firing of action potentials induced by depolarizing steps of current injected across the membrane.
Chemical properties
Off-White Powder
Originator
Dainippon (Japan)
The Uses of Zonisamide
anticonvulsant;carbonic anhydrase inhibitor, repetitive firing of voltage-gated sodium channels and reduction of T-type calcium channel currents blocker
The Uses of Zonisamide
Sulfonamide antiseizure agent; blocks repetitive firing of voltagesensitive sodium channels and reduces voltage-sensitive T-type calcium currents. Heterocyclic methanesulfonide with anticonvulsant pro perties. The compound is under investigation for potential therapeutic use as an antiepileptic drug. Anticonvulsant.
The Uses of Zonisamide
muscarinic antagonist used as an antispasmodic
The Uses of Zonisamide
For use as adjunctive treatment of partial seizures in adults with epilepsy.
Background
Zonisamide is a sulfonamide anticonvulsant used as an adjunctive therapy in adults with partial-onset seizures. Zonisamide may act by blocking repetitive firing of voltage-gated sodium channels, leading to a reduction of T-type calcium channel currents or by binding allosterically to GABA receptors. This latter action may inhibit the uptake of the inhibitory neurotransmitter GABA while enhancing the uptake of the excitatory neurotransmitter glutamate. Zonisamide represents an alternative for patients that remain refractory to established antiepileptic drugs. In 1989, it was approved for commercial use in Japan. The US and Europe approved it in 2000 and 2005, respectively.
Indications
Zonisamide capsules are indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy. Zonisamide oral suspension is indicated as adjunctive therapy for the treatment of partial-onset seizures in adults and pediatric patients 16 years of age and older.
Definition
ChEBI: A 1,2-benzoxazole compound having a sulfamoylmethyl substituent at the 3-position.
Manufacturing Process
To a solution of 8.0 of 3-bromomethyl-1,2-benzisoxazole (m.p. 64-66°) in 130
ml of methanol was added a solution of 8.1 g of sodium sulfite in 130 ml of
water. The mixture was heated with stirring at 50°C for 4 hours and
concentrated under reduced pressure. The crystalline residue was dissolved in
250 ml of methanol with warming and the insoluble material was filtered off.
The filtrate was concentrated under reduced pressure and the crystalline
residue of 1,2-benzisoxazole-3-methanesulfonyl chloride was washed with
diethyl ether to give crude sodium 1,2-benzisoxazole-3-methanesulfonate
(10.5 g).
To 100 ml of phosphorus oxychloride was added 10.5 g of the above-
mentioned sodium salt and the mixture was heated under reflux for 3 hours.
The excess of phosphorus oxychloride was distilled off under reduced
pressure. The residue was dissolved in 200 ml of ethyl acetate and the
removal of the insoluble material by filtration gave the solution of the 1,2-
benzisoxazole-3-methanesulfonyl chloride.
The solution of 1,2-benzisoxazole-3-methanesulfonyl chloride in ethyl acetate,
was cooled on an ice bath, saturated with dry ammonia gas, and allowed to
stand at room temperature for one hour. After the removal of the insoluble
material by filtration, the filtrate was concentrated to yield a crystalline solid,
which was washed with a small amount of ethyl acetate and recrystallized
from ethyl acetate to give the 3-sulfamoylmethyl-1,2-benzisoxazole (5.2 g),
m.p. 160-163°C.
brand name
Zonegran (Dainippon Pharmaceutical Co., Japan);Exegran.
Therapeutic Function
Anticonvulsant; Antiepileptic
Biological Functions
Zonisamide has only recently been approved for use in the United States, although it has been available in Japan for several years. It is effective in partial complex and generalized tonic–clonic seizures and also appears to be beneficial in certain myoclonic seizures. It has a long half-life (about 60 hours) and requires about 2 weeks to achieve steady-state levels. It causes cerebellovestibular side effects similar to those of most other AEDs sharing its mechanism of action. In addition, it appears to cause an increased incidence of kidney stones.
General Description
Zonisamide, a sulfonamide-type anticonvulsant was recentlyapproved for adjunctive therapy in the treatment ofpartial seizures in adults with epilepsy.Zonisamide isprimarily metabolized by reductive ring cleavage of the 1,2-benzisoxazole ring to 2-sulfamoyl-acetyl-phenol. This biotransformation is mainly carried out by theintestinal bacteria rather than the mammalian cytosolicaldehyde oxidase suggested earlier.Again, because ofthe presence of a sulfonamide moiety in zonisamide molecule,precaution should be given to patients who have ahistory of hypersensitivity reactions toward sulfonamidedrugs and concomitant use of zonisamide with other carbonicanhydrase inhibitors should also be avoided.
Mechanism of action
Zonisamide is a sulfonamide derivative that is indicated as an adjunct for partial seizures in patients older than 16 years whose seizures are not controlled by first-line drugs. In Japan, it is used for myoclonic seizures as well. Apparently, it has more than one mechanism of action—all as yet unidentified. It is known to produce blockade of both sodium and T-type calcium channels. Because it also affects dopaminergic transmission, bipolar or schizoaffective disorder patients may improve.
Pharmacokinetics
The absorption for orally administered zonisamide is slow but nearly complete. Its
pharmacokinetics are nonlinear, with a half life of 50 to 70 hours when administered alone or 27 to 46 hours when administered
concurrently with enzyme-inducing AEDs. Protein binding is moderate (<50%). An oral dose of zonisamide is completely
absorbed, with peak plasma concentration occurring in 2 to 6 hours. Although the presence of food will delay the attainment of
its peak plasma concentration, oral bioavailability does not appear to be altered. More than one-third of each oral dose is
excreted in the urine in an unchanged form. The routes of metabolism for zonisamide include acetylation to form its N-acetyl
metabolite, reduction by CYP3A4/CYP2D6, and the formation of an open-ring metabolite, 2-sulfamoylacetyl phenol. These
metabolites subsequently are eliminated unconjugated or glucuronidated in the urine, with an elimination half-life of 63 hours.
Its coadministration with enzyme-inducing AEDs, such as phenytoin, CBZ, or phenobarbital, and with valproate will alter its
pharmacokinetics by reducing its half-life and serum concentration. The half-life for zonisamide is decreased to 27 hours in the
presence of phenytoin, to 38 hours in the presence of either CBZ or phenobarbital, and to 46 hours with valproate. Other drugs
that inhibit or induce CYP3A4 could affect the metabolism of zonisamide.
Zonisamide should be used with caution in patients with hepatic or renal disease. It also has shown to be teratogenic in animal
studies.
Clinical Use
Antiepileptic
Side Effects
Zonisamide is contraindicated in patients with a history of allergy to sulfonamides. The most frequent side effects include somnolence, anorexia, dizziness, agitation, confusion, headache, cognitive impairment, and memory loss. In addition, an incidence of drug-induced psychosis has been noted. Reports from both the United States and Europe have indicated that development of renal stones may occur with use of this drug. A family history of nephrolithiasis may be a contraindication, and urinary monitoring for hypercalciuria may be warranted in bedridden patients or those receiving multiple AEDs. Although the incidence of severe rashes attributable to zonisamide is low, sulfonamides are associated with Stevens-Johnson syndrome. Thus, it is recommended to discontinue the drug immediately should a rash occur.
Safety Profile
Moderately toxic by ingestion,intraperitoneal, subcutaneous, and intravenous routes. Anexperimental teratogen. Other experimental reproductiveeffects. When heated to decomposition it emits very toxicfumes of SOx and NOx. An anticonvulsant.
Veterinary Drugs and Treatments
Zonisamide may be useful as an “add-on” drug for refractory epilepsy in dogs.
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: anticonvulsant effect antagonised;
avoid with St John’s wort.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: increased risk of convulsions.
Metabolism
Zonisamide metabolites are generated mainly by principally reductive and conjugative mechanisms. Oxidation reactions play a minor role in the metabolism of zonisamide. Zonisamide is metabolized by N-acetyl-transferases to form N-acetyl zonisamide and reduced to form the open ring metabolite, 2–sulfamoylacetylphenol (SMAP). The reduction of zonisamide to SMAP is mediated by CYP3A4. Zonisamide does not induce liver enzymes or its own metabolism.
Metabolism
Zonisamide is metabolised mainly by reductive cleavage
of the benzisoxazole ring of the parent drug by CYP3A4
to form 2-sulphamoylacetylphenol (SMAP) and also
by N-acetylation. Parent drug and SMAP can also be
glucuronidated.
The metabolites, which could not be detected in
plasma, are inactive. Excretion is mainly in the urine;
about 15 to 30
% appearing as unchanged drug, 15
%
as N-acetylzonisamide, and 50
% as the glucuronide of
SMAP.
Storage
Store at +4°C
Properties of Zonisamide
| Melting point: | 275°C dec. |
| Boiling point: | 223°C (rough estimate) |
| Density | 1.4306 (rough estimate) |
| refractive index | 1.5690 (estimate) |
| Flash point: | 9℃ |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | H2O: >5 mg/mL, soluble |
| form | solid |
| pka | 10.2(at 25℃) |
| color | off-white |
| Merck | 14,10192 |
| CAS DataBase Reference | 68291-97-4(CAS DataBase Reference) |
Safety information for Zonisamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Zonisamide
| InChIKey | UBQNRHZMVUUOMG-UHFFFAOYSA-N |
Zonisamide manufacturer
Jai Radhe Sales
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![BENZO[D]ISOXAZOL-3-YL-METHANESULFONYL CHLORIDE](https://img.chemicalbook.in/CAS/GIF/73101-65-2.gif)
You may like
-
 68291-97-4 Zonisamide 98%View Details
68291-97-4 Zonisamide 98%View Details
68291-97-4 -
 ZONISAMIDE 99%View Details
ZONISAMIDE 99%View Details
182426-97-9 -
 Zonisamide CAS 68291-97-4View Details
Zonisamide CAS 68291-97-4View Details
68291-97-4 -
 Zonisamide 98.00% CAS 68291-97-4View Details
Zonisamide 98.00% CAS 68291-97-4View Details
68291-97-4 -
 Zonisamide CAS 68291-97-4View Details
Zonisamide CAS 68291-97-4View Details
68291-97-4 -
 Zonisamide CAS 68291-97-4View Details
Zonisamide CAS 68291-97-4View Details
68291-97-4 -
 99% Zonisamide Powder API MANUFACTURER INDIA, 25KG, Non prescriptionView Details
99% Zonisamide Powder API MANUFACTURER INDIA, 25KG, Non prescriptionView Details
68291-97-4 -
 Zonisamide Usp ApiView Details
Zonisamide Usp ApiView Details
68291-97-4
