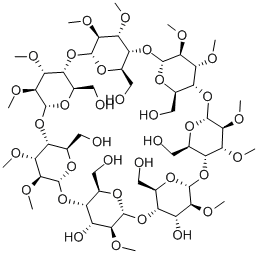
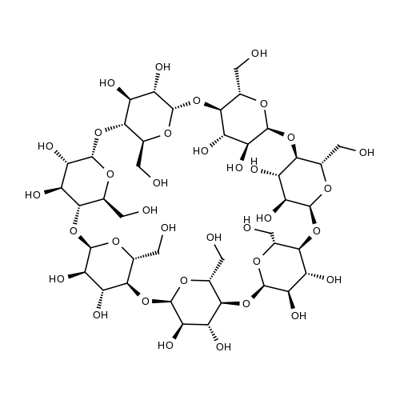
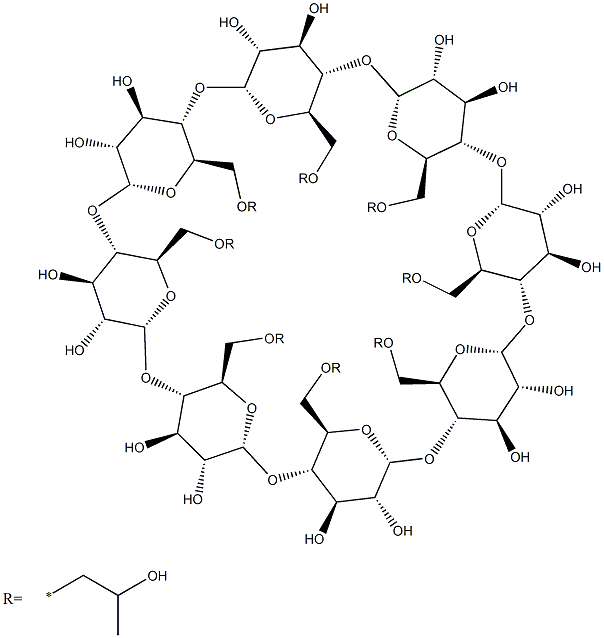
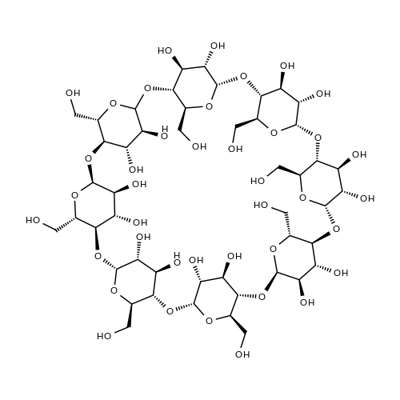
Betadex Sulfobutyl Ether Sodium
- CAS NO.:182410-00-0
- Empirical Formula: C42H70O35
- Molecular Weight: 1134.9842
- MDL number: MFCD12032209
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-06 09:34:49
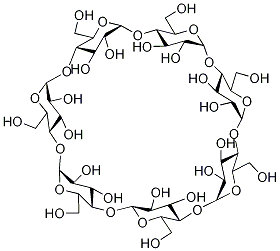
What is Betadex Sulfobutyl Ether Sodium?
Chemical properties
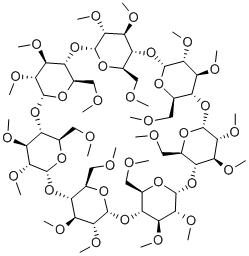
β-Cyclodextrin, sulfobutyl ethers, sodium salts is a cyclic oligosaccharide containing seven D-(t)- glucopyranose units attached by α(1→4) glucoside bonds. Sulfobutylether b-cyclodextrin is an anionic bcyclodextrin derivative with a sodium sulfonate salt separated from the hydrophobic cavity by a butyl spacer group. The substituent is introduced at positions 2, 3, and 6 in at least one of the glucopyranose units in the cyclodextrin structure. Introducing the sulfobutylether (SBE) into b-cyclodextrin can produce materials with different degrees of substitution, theoretically from 1 to 21; the hepta-substituted preparation (SBE7-b-CD) is the cyclodextrin with the most desirable drug carrier properties. Sulfobutylether b-cyclodextrin occurs as a white amorphous powder.
The Uses of Betadex Sulfobutyl Ether Sodium
β-Cyclodextrin, sulfobutyl ethers, sodium salts with sulfobutyl ether groups and sodium ions substituted along the length of the molecule. β-Cyclodextrin is a cyclic oligosaccharide produced from starch via enzymatic conversion. β-Cyclodextrin, sulfobutyl ethers, sodium salts is commonly used to produce HPLC columns allowing chiral enantiomers separation.
The Uses of Betadex Sulfobutyl Ether Sodium
SBE- β –CD is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The Uses of Betadex Sulfobutyl Ether Sodium
β-Cyclodextrin with sulfobutyl ether groups and sodium ions substituted along the length of the molecule. β-Cyclodextrin is a cyclic oligosaccharide produced from starch via enzymatic conversion. β-Cyclodextrin is commonly used to produce HPLC columns allowing chiral enantiomers separation.
Production Methods
Sulfobutylether β-cyclodextrin is prepared by alkylation of bcyclodextrin using 1,4-butane sultone under basic conditions. The degree of substitution in b-cyclodextrin is controlled by the stoichiometric ratio of β-cyclodextrin to sultone used in the process.
Pharmaceutical Applications
Cyclodextrins are crystalline, nonhygroscopic, cyclic oligosaccharides
derived from starch (see Cyclodextrins). Sulfobutylether bcyclodextrin
is an amorphous, anionic substituted b-cyclodextrin
derivative; other substituted cyclodextrin derivatives
are also available.
Sulfobutylether b-cyclodextrin can form noncovalent complexes
with many types of compounds including small organic molecules,
peptides, and proteins. It can also enhance their solubility
and stability in water. The first application of sulfobutylether bcyclodextrin
was in injectable preparations; it can also be used in
oral solid and liquid dosage forms, and ophthalmic,
inhalation, and intranasal formulations. Sulfobutylether bcyclodextrin
can function as an osmotic agent and/or a solubilizer
for controlled-release delivery, and has antimicrobial preservative
properties when present at sufficient concentrations.
The amount of sulfobutylether β-cyclodextrin that may be used
is dependent on the purpose for inclusion in the formulation, the
route of administration, and the ability of the cyclodextrin to
complex with the drug being delivered. The minimum amount
required for solubilization is, in general, a cyclodextrin/drug molar
ratio of approximately 1–5 (the exact ratio being experimentally
determined from complexation data). The maximum use in a
formulation may be limited by physicochemical constraints such as
viscosity (e.g. syringeable concentrations may be considered up to
50% w/v), tonicity, or the total weight and size of solid dosage
forms (e.g. less than a gram in an individual tablet). It may also be
limited by pharmacokinetic/pharmacodynamic (PK/PD) considerations.
As dilution of a cyclodextrin formulation leads to an increase
in the amount of uncomplexed drug, formulations that are not
diluted upon administration, such as ophthalmic formulations, are
sensitive to cyclodextrin concentration. In formulations such as
these, cyclodextrin concentrations greater than the minimum
required for solubilization can reduce the availability of uncomplexed
drug and thereby affect PK/PD expectations by producing
effects such as slower onset, lower Cmax, and bioavailability.
Toxicity evaluation
Sulfobutylether b-cyclodextrin sodium (SBECD) has been associated with toxic effects in the kidney. Doses up to 1500 mg/kg produced no histopathological evidence of toxicity in dog kidneys.
Safety
Sulfobutylether b-cyclodextrin is derived from b-cyclodextrin,
which is nephrotoxic when administered parenterally.
However, studies have shown that sulfobutylether bcyclodextrin
is well tolerated at high doses, when administered via
intravenous bolus injections, orally, and by inhalation. Up to
9 g/day may be administered by IV infusion in a licensed
voriconazole formulation. The safety following high doses of
sulfobutylether β-cyclodextrin intravenous administration in
humans is continually being investigated.
Sulfobutylether β-cyclodextrin has been subjected to an extensive
battery of in vitro and in vivo genotoxicity and pharmacological
evaluations. No genotoxic or mutagenic changes were
observed with sulfobutylether β-cyclodextrin administration. Sulfobutylether
β-cyclodextrin is biocompatible and exhibits no
pharmacological activity. It is rapidly eliminated unmetabolized
when administered intravenously.
storage
Sulfobutylether b-cyclodextrin(182410-00-0) is stable in the solid state and should be protected from high humidity. It should be stored in a tightly sealed container in a cool, dry place.
It will reversibly take up moisture without any effect on the appearance of the material at humidities up to 60% RH. Equilibration at RH values above 60% will result in deliquescence. Once in this state, the material can be dried, but will give a glasslike product. This water absorption behavior is typical of amorphous hygroscopic materials.
Sulfobutylether b-cyclodextrin is stable in aqueous solutions at values above about pH 1. It can degrade in highly acidic (pH < 1) solutions, particularly at elevated temperatures, producing the ring-opened form, followed by hydrolysis of the a(1-4) glucoside bonds.
Sulfobutylether b-cyclodextrin solutions may be autoclaved.
Incompatibilities
The preservative activity of benzalkonium chloride is reduced in the presence of sulfobutylether b-cyclodextrin.
Regulatory Status
Sulfobutylether β-cyclodextrin is included in IV and IM injectable products currently approved and marketed in the USA, Europe, and Japan. It is included in the FDA Inactive Ingredients Database for IM and IV use. Its use by other routes, including SC, oral, inhalation, nasal and ophthalmic, is being evaluated in clinical studies.
Properties of Betadex Sulfobutyl Ether Sodium
| Melting point: | 202-204°C (dec.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in water.
Insoluble in acetone, methanol, chloroform. |
| form | Solid powder |
| color | White to off white |
Safety information for Betadex Sulfobutyl Ether Sodium
Computed Descriptors for Betadex Sulfobutyl Ether Sodium
| InChIKey | WHGYBXFWUBPSRW-CQFZCEJCNA-N |
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 182410-00-0 / 194615-04-8 Sulfobutylether beta-cyclodextrin sodium salt 99%View Details
182410-00-0 / 194615-04-8 Sulfobutylether beta-cyclodextrin sodium salt 99%View Details
182410-00-0 / 194615-04-8 -
 182410-00-0 / 194615-04-8 98%View Details
182410-00-0 / 194615-04-8 98%View Details
182410-00-0 / 194615-04-8 -
 Sulfobutyl ether beta-cyclodextrin sodium, 99% CAS 182410-00-0View Details
Sulfobutyl ether beta-cyclodextrin sodium, 99% CAS 182410-00-0View Details
182410-00-0 -
 Sodium sulphobutylether-beta-cyclodextrin 97.00% CAS 182410-00-0View Details
Sodium sulphobutylether-beta-cyclodextrin 97.00% CAS 182410-00-0View Details
182410-00-0 -
 Sodium sulphobutylether-beta-cyclodextrin 96% CAS 182410-00-0View Details
Sodium sulphobutylether-beta-cyclodextrin 96% CAS 182410-00-0View Details
182410-00-0 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6
