gamma-Cyclodextrin
Synonym(s):γ-Cyclodextrin;Cyclomaltooctaose;Cyclooctaamylose;Schardinger γ-Dextrin
- CAS NO.:17465-86-0
- Empirical Formula: C48H80O40
- Molecular Weight: 1297.12
- MDL number: MFCD00009595
- EINECS: 241-482-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
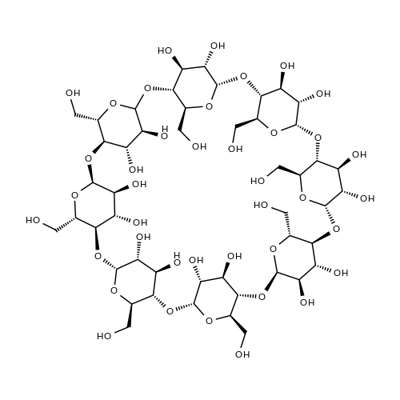
What is gamma-Cyclodextrin ?
Chemical properties
White powder or crystal
Chemical properties
Cyclodextrins occur as white, practically odorless, fine crystalline powders, having a slightly sweet taste. Some cyclodextrin derivatives occur as amorphous powders.
The Uses of gamma-Cyclodextrin
A molecule used to solublize non-polar molecules such a cholesterol for use in cell culture.
The Uses of gamma-Cyclodextrin
γ-Cyclodextrin can be used as a precursor for the synthesis of:
- Octakis-(2,3-di-O-methyl-6-O-carboxymethyl)-γ-cyclodextrin sodium salt (ODMCM). ODMCM is used in capillary electrophoresis as a chiral resolving agent.
- Water-soluble cyclodextrin thioethers derivatives for the transportation of hydrophobic drugs.
The Uses of gamma-Cyclodextrin
γ-Cyclodextrin (γ-CD), a water-soluble cyclic oligosaccharide consisting of eight α-(1,4)-linked glucopyranose subunits, can be used in a wide range of applications including:
- Synthesis of renewable and biocompatible metal-organic frameworks.
- Formation of inclusion complexes with a variety of guest molecules to increase their solubility in water and other polar solvents.
- Synthesis of γ-CD based nanogels and dendrimers as carriers and stabilizers for drug delivery applications.
Definition
ChEBI: Gamma-cyclodextrin is a cycloamylose composed of eight alpha-(1->4) linked D-glucopyranose units.
What are the applications of Application
γ-Cyclodextrin is a cyclic oligosaccharide
Production Methods
Cyclodextrins are manufactured by the enzymatic degradation of starch using specialized bacteria. For example, β-cyclodextrin is produced by the action of the enzyme cyclodextrin glucosyltransferase upon starch or a starch hydrolysate. An organic solvent is used to direct the reaction that produces β-cyclodextrin, and to prevent the growth of microorganisms during the enzymatic reaction. The insoluble complex of β-cyclodextrin and organic solvent is separated from the noncyclic starch, and the organic solvent is removed in vacuo so that less than 1 ppm of solvent remains in the β-cyclodextrin. The β-cyclodextrin is then carbon treated and crystallized from water, dried, and collected.
General Description
Cyclodextrins belongs to the family of cyclic α-1,4-glucans and is made of glucose units. This molecule has a shape of a hollow cone. It possesses a hydrophilic external and hydrophobic internal surface, because of which cyclodextrins can form inclusion complexes. γ-Cyclodextrin is extensively used in food and pharmaceutical industries. Cyclodextrins are obtained by the enzymatic conversion of starch/starch derivatives by cyclodextrin glycosyltransferase.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Cyclodextrins are ‘bucketlike’ or ‘conelike’ toroid molecules, with a rigid structure and a central cavity, the size of which varies according to the cyclodextrin type. The internal surface of the cavity is hydrophobic and the outside of the torus is hydrophilic; this is due to the arrangement of hydroxyl groups within the molecule. This arrangement permits the cyclodextrin to accommodate a guest molecule within the cavity, forming an inclusion complex.
Cyclodextrins may be used to form inclusion complexes with a variety of drug molecules, resulting primarily in improvements to dissolution and bioavailability owing to enhanced solubility and improved chemical and physical stability.
Cyclodextrin inclusion complexes have also been used to mask the unpleasant taste of active materials and to convert a liquid substance into a solid material. γ-cyclodextrin has the largest cavity and can be used to form inclusion complexes with large molecules;it has low toxicity and enhanced water solubility.
In parenteral formulations, cyclodextrins have been used to produce stable and soluble preparations of drugs that would otherwise have been formulated using a nonaqueous solvent.
In eye drop formulations, cyclodextrins form water-soluble complexes with lipophilic drugs such as corticosteroids. They have been shown to increase the water solubility of the drug; to enhance drug absorption into the eye; to improve aqueous stability; and to reduce local irritation.
Cyclodextrins have also been used in the formulation of solutions,suppositories, and cosmetics.
Safety
Cyclodextrins are starch derivatives and are mainly used in oral and
parenteral pharmaceutical formulations. They are also used in
topical and ophthalmic formulations.
Cyclodextrins are also used in cosmetics and food products, and
are generally regarded as essentially nontoxic and nonirritant
materials. However, when administered parenterally, β-cyclodextrin
is not metabolized but accumulates in the kidneys as insoluble
cholesterol complexes, resulting in severe nephrotoxicity.
Cyclodextrin administered orally is metabolized by microflora in
the colon, forming the metabolites maltodextrin, maltose, and
glucose; these are themselves further metabolized before being
finally excreted as carbon dioxide and water. Although a study
published in 1957 suggested that orally administered cyclodextrins
were highly toxic, more recent animal toxicity studies in rats and
dogs have shown this not to be the case, and cyclodextrins are now
approved for use in food products and orally administered
pharmaceuticals in a number of countries.
Cyclodextrins are not irritant to the skin and eyes, or upon
inhalation. There is also no evidence to suggest that cyclodextrins
are mutagenic or teratogenic.
γ-Cyclodextrin
LD50 (rat, IP): 4.6 g/kg
LD50 (rat ,IV): 4.0 g/kg
LD50 (rat, oral): 8.0 g/kg
Storage
Cyclodextrins should be stored in a tightly sealed container, in a cool, dry place.Cyclodextrins are stable in the solid state if protected from high humidity.
Regulatory Status
Included in the FDA Inactive Ingredients Database: α-cyclodextrin
(injection preparations); β-cyclodextrin (oral tablets, topical gels);
γ-cyclodextrin (IV injections).
Included in the Canadian List of Acceptable Non-medicinal
Ingredients (stabilizing agent; solubilizing agent ); and in oral and
rectal pharmaceutical formulations licensed in Europe, Japan, and
the USA.
Properties of gamma-Cyclodextrin
| Melting point: | ≥300 °C |
| Boiling point: | 845.2°C (rough estimate) |
| alpha | [α]D25 +174~+179° (c=1, H2O) (After Drying) |
| Density | 1.2064 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| Flash point: | 450℃ |
| storage temp. | room temp |
| solubility | 1 M NaOH: 25 mg/mL, may be clear to slightly hazy |
| form | powder |
| pka | 11.68±0.70(Predicted) |
| color | white |
| Odor | at 100.00?%. odorless |
| optical activity | [α]/D 174.0 to 180.0° |
| Water Solubility | 232g/L(25 ºC) |
| λmax | λ: 420 nm Amax: ≤0.20 |
| Merck | 14,2718 |
| BRN | 5725162 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | .gamma.-Cyclodextrin (17465-86-0) |
Safety information for gamma-Cyclodextrin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for gamma-Cyclodextrin
| InChIKey | GDSRMADSINPKSL-HSEONFRVSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 17465-86-0 Gamma Cyclodextrien 98%View Details
17465-86-0 Gamma Cyclodextrien 98%View Details
17465-86-0 -
 γ-Cyclodextrin CAS 17465-86-0View Details
γ-Cyclodextrin CAS 17465-86-0View Details
17465-86-0 -
 γ-Cyclodextrin, ≥98% CAS 17465-86-0View Details
γ-Cyclodextrin, ≥98% CAS 17465-86-0View Details
17465-86-0 -
 Gamma-Cyclodextrin CAS 17465-86-0View Details
Gamma-Cyclodextrin CAS 17465-86-0View Details
17465-86-0 -
 Gamma Cyclodextrin CAS 17465-86-0View Details
Gamma Cyclodextrin CAS 17465-86-0View Details
17465-86-0 -
 GAMMA-CYCLODEXTRIN (IMPURITY) CAS 17465-86-0View Details
GAMMA-CYCLODEXTRIN (IMPURITY) CAS 17465-86-0View Details
17465-86-0 -
 Gamma-cyclodextrin 98% CAS 17465-86-0View Details
Gamma-cyclodextrin 98% CAS 17465-86-0View Details
17465-86-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
