β-Cyclodextrin
Synonym(s):beta-Cyclodextrin;β-Cyclodextrin;Caraway;Cavamax W7;Cavamax W7 Pharma
- CAS NO.:7585-39-9
- Empirical Formula: C42H70O35
- Molecular Weight: 1134.99
- MDL number: MFCD00078139
- EINECS: 231-493-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 22:22:14
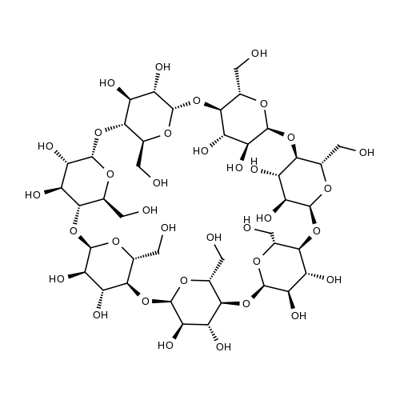
What is β-Cyclodextrin?
Description
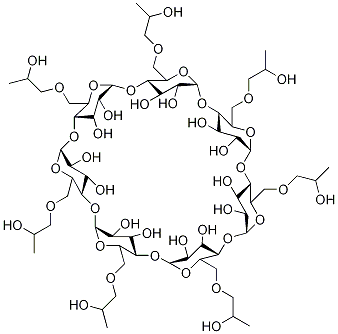
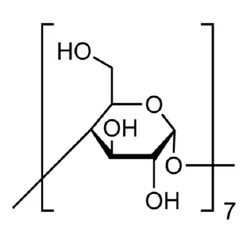
Cyclodextrins are sugar molecules bound together in rings of various sizes. Specifically, the sugar units are called glucopyranosides—glucose molecules that exist in the pyranose (six-membered) ring configuration.?Six, seven, or eight glucopyranosides bind with each other to form α-, β-, and γ-cyclodextrin, respectively. β-Cyclodextrin is shown in the images.
The three cyclodextrins occur naturally; they were first described in 1891 by A. Villiers, who called them “cellulosine”. A few years later, A. Schardinger characterized the three variants.
As complex as they are, cyclodextrins are relatively easy to make. Starch is treated with a combination of common enzymes, notably cyclodextrin glycosyl transferase and α-amylase. Each enzyme combination produces a characteristic ratio of the three cyclodextrins.
Cyclodextrins form a toroid (truncated cone) configuration with multiple hydroxyl groups at each end. This allows them to encapsulate hydrophobic compounds without losing their solubility in water.
Among other applications, cyclodextrins can be used to carry hydrophobic drug molecules into biological systems. Cyclodextrins used in the laundry product Febreze trap odor molecules so that they do not reach the scent receptors in your nose.
Chemical properties
white powder
Chemical properties
Cyclodextrins occur as white, practically odorless, fine crystalline powders, having a slightly sweet taste. Some cyclodextrin derivatives occur as amorphous powders.
Chemical properties
A biennial herbaceous plant very common in Europe, Asia, Africa and the United States, it has a tapering fleshy root, furrowed stem, finely cut feathery leaves, umbels of small flower heads in midsummer, and capsules containing two curved narrow seeds. The plant grows to about 60 cm and it blooms from May to July. The part used is the fruit, containing approximately 15% of fixed oils and 3 to 7% of essential oil. Caraway has a warm, biting flavor with a strong, fatty, harsh undernote.
Chemical properties
beta Cyclodextrin is a virtually odorless, slightly sweet-tasting, white or almost white crystalline solid or fine powder.
Occurrence
A derivative of naturally occurring starch.
The Uses of β-Cyclodextrin
β-Cyclodextrin is a cyclic oligosaccharide produced from starch via enzymatic conversion. β-Cyclodextrin is commonly used to produce HPLC columns allowing chiral enantiomers separation.
The Uses of β-Cyclodextrin
Use to solubilize non-polar compounds such as fatty acids, lipids and cholesterol. Reported useful for the selective precipitation of enantiomeric, positional or structural isomersβ-Cyclodextrin is used with dansyl chloride to form water-soluble complexes for fluorescent labeling of proteins. It is an active ingredient of household odor eliminator. It is also used in personal care products like toothpastes, skin creams and dusting powders. It finds applications in the cosmetic industry for products like detergents and perfumes for the controlled release of fragrances. Further, it is used to produce HPLC columns allowing chiral enantiomers separation. In addition to this, it is used to decrease the level of cholesterol in milk fat.
What are the applications of Application
β-Cyclodextrin is useful for forming water-soluble complexes for fluorescent labeling of proteins
Production Methods
Cyclodextrins are manufactured by the enzymatic degradation of starch using specialized bacteria. For example, β-cyclodextrin is produced by the action of the enzyme cyclodextrin glucosyltransferase upon starch or a starch hydrolysate. An organic solvent is used to direct the reaction that produces β-cyclodextrin, and to prevent the growth of microorganisms during the enzymatic reaction. The insoluble complex of β-cyclodextrin and organic solvent is separated from the noncyclic starch, and the organic solvent is removed in vacuo so that less than 1 ppm of solvent remains in the β-cyclodextrin. The β-cyclodextrin is then carbon treated and crystallized from water, dried, and collected.
Preparation
Usually produced commercially from Bacillus macerans or B. circulans fermentation of starch or starch hydrolysate.
Definition
ChEBI: Beta-cyclodextrin is a cyclodextrin composed of seven alpha-(1->4) linked D-glucopyranose units.
Essential oil composition
In addition to carvone, the oil contains d-limonene, carveol, diacetyl furfural, methyl alcohol, acetic aldehyde and other substances. Caraway oil consists of 3.5 to 7% volatile and fatty oils; resin, sugar, tannin, mucilage.
Taste threshold values
Reported to have a taste threshold value lower than that of sucrose with a detection level of 3.9 to 27 ppm and a recognition level of 11 to 52 ppm
General Description
Beta-Cyclodextrin is the most abundant and cheap cyclic oligosaccharide that forms inclusion complexes with several drug molecules. Its main application is in tablet and capsule formulations.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Cyclodextrins are ‘bucketlike’ or ‘conelike’ toroid molecules, with a rigid structure and a central cavity, the size of which varies according to the cyclodextrin type. The internal surface of the cavity is hydrophobic and the outside of the torus is hydrophilic; this is due to the arrangement of hydroxyl groups within the molecule. This arrangement permits the cyclodextrin to accommodate a guest molecule within the cavity, forming an inclusion complex.
Cyclodextrins may be used to form inclusion complexes with a variety of drug molecules, resulting primarily in improvements to dissolution and bioavailability owing to enhanced solubility and improved chemical and physical stability.
Cyclodextrin inclusion complexes have also been used to mask the unpleasant taste of active materials and to convert a liquid substance into a solid material.
b-Cyclodextrin is the most commonly used cyclodextrin, although it is the least soluble. It is the least expensive cyclodextrin; is commercially available from a number of sources; and is able to form inclusion complexes with a number of molecules of pharmaceutical interest. However, b-cyclodextrin is nephrotoxic and should not be used in parenteral formulations. b-Cyclodextrin is primarily used in tablet and capsule formulations.
In oral tablet formulations, b-cyclodextrin may be used in both wet-granulation and direct-compression processes. The physical properties of b-cyclodextrin vary depending on the manufacturer. However, b-cyclodextrin tends to possess poor flow properties and requiresalubricant,such as 0.1% w/w magnesium stearate,when it is directly compressed.
In parenteral formulations, cyclodextrins have been used to produce stable and soluble preparations of drugs that would otherwise have been formulated using a nonaqueous solvent.
In eye drop formulations, cyclodextrins form water-soluble complexes with lipophilic drugs such as corticosteroids. They have been shown to increase the water solubility of the drug; to enhance drug absorption into the eye; to improve aqueous stability; and to reduce local irritation.
Cyclodextrins have also been used in the formulation of solutions,suppositories, and cosmetics.
Biochem/physiol Actions
β-Cyclodextrin is the cyclic α heptamer of glucose. It acts as a host to form inclusion compounds with the guests including derivatives of benzene, cyclohexane, adamantane, other alicyclic guests, and also inorganic molecules or ions. It is generally used to solubilize non-polar compounds such a fatty acids, lipids and cholesterol.
Safety
Cyclodextrins are starch derivatives and are mainly used in oral and
parenteral pharmaceutical formulations. They are also used in
topical and ophthalmic formulations.
Cyclodextrins are also used in cosmetics and food products, and
are generally regarded as essentially nontoxic and nonirritant
materials. However, when administered parenterally, β-cyclodextrin
is not metabolized but accumulates in the kidneys as insoluble
cholesterol complexes, resulting in severe nephrotoxicity.
Cyclodextrin administered orally is metabolized by microflora in
the colon, forming the metabolites maltodextrin, maltose, and
glucose; these are themselves further metabolized before being
finally excreted as carbon dioxide and water. Although a study
published in 1957 suggested that orally administered cyclodextrins
were highly toxic, more recent animal toxicity studies in rats and
dogs have shown this not to be the case, and cyclodextrins are now
approved for use in food products and orally administered
pharmaceuticals in a number of countries.
Cyclodextrins are not irritant to the skin and eyes, or upon
inhalation. There is also no evidence to suggest that cyclodextrins
are mutagenic or teratogenic.
β-Cyclodextrin
LD50 (mouse, IP): 0.33 g/kg(16)
LD50 (mouse, SC): 0.41 g/kg
LD50 (rat, IP): 0.36 g/kg
LD50 (rat, IV): 1.0 g/kg
LD50 (rat, oral): 18.8 g/kg
LD50 (rat, SC): 3.7 g/kg
Storage
Cyclodextrins should be stored in a tightly sealed container, in a cool, dry place.Cyclodextrins are stable in the solid state if protected from high humidity.
Purification Methods
Recrystallise β-cyclodextrin from water and dry it for 12hours in a vacuum at 110o, or 24hours in a vacuum at 70o. The purity is assessed by TLC on cellulose containing a fluorescent indicator. [Taguchi, J Am Chem Soc 108 2705 1986, Tabushi et al. J Am Chem Soc 108 4514 1986, Orstam & Ross J Phys Chem 91 2739 1987.] [Beilstein 19 IV 6287, 19/12 V 801.]
Regulatory Status
Included in the FDA Inactive Ingredients Database: α-cyclodextrin
(injection preparations); β-cyclodextrin (oral tablets, topical gels);
γ-cyclodextrin (IV injections).
Included in the Canadian List of Acceptable Non-medicinal
Ingredients (stabilizing agent; solubilizing agent ); and in oral and
rectal pharmaceutical formulations licensed in Europe, Japan, and
the USA.
Properties of β-Cyclodextrin
| Melting point: | 290-300 °C (dec.) (lit.) |
| Boiling point: | 844.96°C (rough estimate) |
| alpha | [α]D25 +159~+165° (c=1, H2O) (After Drying) |
| Density | 1.2296 (rough estimate) |
| FEMA | 4028 | BETA-CYCLODEXTRIN |
| refractive index | 1.7500 (estimate) |
| storage temp. | room temp |
| solubility | 1 M NaOH: 50 mg/mL |
| form | powder |
| pka | 11.73±0.70(Predicted) |
| color | white |
| PH | 5.0-8.0 (1% in solution, Ph Eur) |
| Odor | at 100.00 %. odorless |
| optical activity | [α]20/D +162±3°, c = 1.5% in H2O |
| Water Solubility | Soluble in water and ammonium hydroxide. |
| Merck | 14,2718 |
| BRN | 78623 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | .beta.-Cyclodextrin (7585-39-9) |
Safety information for β-Cyclodextrin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for β-Cyclodextrin
β-Cyclodextrin manufacturer
Gangwal Healthcare Pvt Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 ß-Cyclodextrin CAS 7585-39-9View Details
ß-Cyclodextrin CAS 7585-39-9View Details
7585-39-9 -
 Beta Cyclodextrin Powder, Grade Standard: IPView Details
Beta Cyclodextrin Powder, Grade Standard: IPView Details
7585-39-9 -
 Beta CyclodextrinView Details
Beta CyclodextrinView Details
7585-39-9 -
 Beta Cyclodextrin Powder, Grade Standard: IPView Details
Beta Cyclodextrin Powder, Grade Standard: IPView Details
7585-39-9 -
 Beta-Cyclodextrin (BCD), 25KgView Details
Beta-Cyclodextrin (BCD), 25KgView Details
7585-39-9 -
 Beta Cyclodextrin Chemical, 20kgView Details
Beta Cyclodextrin Chemical, 20kgView Details
7585-39-9 -
 BETA CYCLODEXTRINView Details
BETA CYCLODEXTRINView Details
7585-39-9 -
 Beta Cyclodextrin Chemical, 25KgView Details
Beta Cyclodextrin Chemical, 25KgView Details
7585-39-9
