13597-99-4
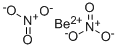
Product Name:
Beryllium nitrate
Formula:
BeN2O6
Synonyms:
Beryllium nitrate solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Beryllium nitrate is a white to pale yellow crystalline solid. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. If large quantities of the material are involved or the material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used in chemical analysis. |
|---|---|
| Color/Form | White solid |
| Odor | Odorless |
| Melting Point | 60 °C; decomposes at 100-200 °C |
| Solubility | Soluble in water |
| Density | 1.56 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Stable under recommended storage conditions. /Beryllium nitrate solution/ |
| Decomposition | 100 °C |
| Corrosivity | In presence of moisture will damage wood and corrode most metals. |
| Other Experimental Properties | On heating above 100 °C, beryllium nitrate decomposes with simultaneous loss of water and oxides to nitrogen. Decomposition is complete above 250 °C. |
| Chemical Classes | Metals -> Beryllium Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 133.02 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 132.9878188 g/mol |
| Monoisotopic Mass | 132.9878188 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Beryllium nitrate is a white to pale yellow crystalline solid. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. If large quantities of the material are involved or the material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used in chemical analysis.