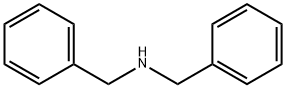
Benzylamine
Synonym(s):α-Aminotoluene;Benzylamine;Phenylmethylamine
- CAS NO.:100-46-9
- Empirical Formula: C7H9N
- Molecular Weight: 107.15
- MDL number: MFCD00008106
- EINECS: 202-854-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
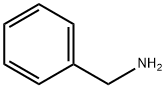
What is Benzylamine?
Chemical properties
colourless liquid with an ammoniacal odour
The Uses of Benzylamine
Benzylamine is used as a chemical intermediate for dyes, pharmaceuticals, and polymers.It is also employed as a corrosion inhibitor and as a brightener in electroplating baths. It also finds use in the manufacture of explosives.
The Uses of Benzylamine
Benzylamine is a valuable intermediate for various applications and a building block for chemical synthesis used in pharmaceuticals and crop protection agents. It finds application in the coating industry. It is involved as a carrier electrolyte for separation of alkali, alkaline earth and ammonium cations in water samples by capillary electrophoresis with indirect UV detection. It is also used in synthesis of cross-linked porous copolymer supports based on N-(p-vinylbenzoyl)-2-methylalanine, styrene and divinylbenzene. In the textile industry, it is used in colored dyes. It is often used for medicinal purposes in topical creams and antifungal solutions as well as in vitamins.
The Uses of Benzylamine
Benzylamine may be used as a derivatization agent to increase the sensitivity of 5-hydroxyindoles, catecholamines and catechols in biological samples prior to their determination using high performance liquid chromatography (HPLC) coupled with fluorescence detection.
Definition
ChEBI: A primary amine compound having benzyl as the N-substituent. It has been isolated from Moringa oleifera (horseradish tree).
Preparation
28% Aqueous Ammonia, 810g
Benzyl chloride, 84.3g
49% Aqueous NaOH, 52.3g
Diethyl Ether, 200g
A three-necked flask was equipped with a reflux condenser dropping funnel and an agitator. All of the aqueous ammonium hydroxide solution was poured into the flask, then the benzyl chloride was introduced drop by drop; over a period of two hours with constant stirring of the mixture. The exothermic heat of reaction kept the temperature between 30-34°C during this period. When it is desired to introduce the benzyl chloride at a faster rate, conventional cooling means can be employed to control the temperature. A large excess of ammonia was employed as the molar ratio of reactants was 20:1. An additional two hours was allowed to insure completion of the following reaction:
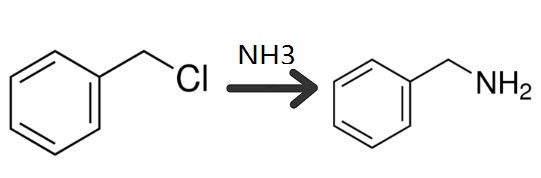
Then the equimolecular quantity of caustic soda solution was added. When quiescent, the mixture split into an aqueous layer and an oily layer. After separating these two layers by use of a separatory funnel, the aqueous phase was made available for further repeated use by merely adding sufficient ammonium hydroxide to bring the total quantity of ammonia up to the original figure. The oily layer was steam distilled until no further oily constituent was visible in the condensed distillate as it dripped down the condenser tube. This distillate was saturated with sodium chloride and successively extracted with an initial 80 and three 40 gram batches of ethyl ether. Upon evaporation of the ether from the extract, 52g of crude benzylamine remain which was distilled at atmospheric pressure. 41g grams of substantially pure benzylamine distilled over in the boiling range 185-192°C, while the residue was found to contain an additional 2.3g of benzylamine and 8.7g of other matter which was believed to consist entirely of dibenzyl amine. Thus the total yield of benzylamine was 43.3g or 60.7% based on the weight of benzyl chloride.
Synthesis Reference(s)
Synthetic Communications, 25, p. 863, 1995 DOI: 10.1080/00397919508013422
Synthesis, p. 48, 1987 DOI: 10.1055/s-1987-27838
General Description
Colorless to light yellow liquid with a strong odor of ammonia. Floats and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
In presence of moisture, Benzylamine may weakly corrode some metals. Liquid will attack some plastics [USCG, 1999]. Neutralize acids to form salts plus water in exothermic reactions. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides.
Hazard
Highly toxic, strong irritant to skin and mucous membranes.
Health Hazard
Inhalation of vapor causes irritation of the mucous membranes of the nose and throat, and lung irritation with respiratory distress and cough. Headache, nausea, faintness, and anxiety can occur. Exposure to vapor produces eye irritation with lachrymation, conjunctivitis, and corneal edema resulting in halos around lights. Direct local contact with liquid is known to produce severe and sometimes permanent eye damage and skin burns. Vapors may also produce primary skin irritation and dermatitis.
Fire Hazard
Special Hazards of Combustion Products: Toxic nitrogen oxides may form in a fire.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: In presence of moisture may severely corrode some metals. In liquid state this chemical will attack some plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Toxicology
Benzylamine is readily biodegradable. It is harmful in aquatic environment [German class for hazards in aquatic environment: 1 (weak effect) (WGK 1)].Acute toxicity: Pseudomonas fluorescens (bacteria) 500 mg (EC0); Scenedesmus quadricauda (algae) 6 mg (96-h EC10); Daphnia magna 60 mg (48-h EC50); Leuciscus idus (fish) 20 mg (48-h EC0); Pimephals promelas (fish) 102 mg (96-h EC50).Benzylamine is Ames test negative (no mutagenic effect). Acute oral toxicity: LD50 1130 mg/kg (rat). Acute percutaneous toxicity: LD50 1340 mg/kg (rat). Toxicity class 3 (Switzerland). All rats inhaling benzylamine for 3 h survived a two-week period (full body exposure). After full body exposure for 5h, 17 % died.
storage
Benzylamine is slowly oxidized on contact with air and forms benzalbenzylamine. It also reacts with carbon dioxide to form its solid carbamic acid salt. It therefore should be stored in a tight closed container for not more than one year. For longer storage, a nitrogen blanket is recommended. In Germany the conditions for storage are classified as 8L (VCI-Lagerklasse).
Purification Methods
Dry it with NaOH or KOH, then distil it under N2, through a column packed with glass helices, taking the middle fraction. Also distil it from zinc dust under reduced pressure. The picrate has m 196o (from EtOH), and the p-toluenesulfonamide has m 116o (from MeOH). [Beilstein 12 IV 2155.]
Properties of Benzylamine
| Melting point: | 10 °C (lit.) |
| Boiling point: | 184-185 °C (lit.) |
| Density | 0.981 g/mL at 25 °C (lit.) |
| vapor pressure | 1.2 hPa (20 °C) |
| refractive index | n |
| Flash point: | 140 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: miscible |
| form | Liquid |
| pka | 9.33(at 25℃) |
| color | Clear colorless to slightly yellow |
| Odor | Strong ammonia. |
| PH | 11.4 (100g/l, H2O, 20℃) |
| explosive limit | 0.7-8.2%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,1125 |
| BRN | 741984 |
| Dielectric constant | 4.6(Ambient) |
| Stability: | Combustible. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 100-46-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzylamine(100-46-9) |
| EPA Substance Registry System | Benzylamine (100-46-9) |
Safety information for Benzylamine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benzylamine
| InChIKey | WGQKYBSKWIADBV-UHFFFAOYSA-N |
Benzylamine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Benzylamine, 99% CAS 100-46-9View Details
Benzylamine, 99% CAS 100-46-9View Details
100-46-9 -
 Benzylamine pure CAS 100-46-9View Details
Benzylamine pure CAS 100-46-9View Details
100-46-9 -
 Benzylamine extrapure AR CAS 100-46-9View Details
Benzylamine extrapure AR CAS 100-46-9View Details
100-46-9 -
 Benzylamine 95% CAS 100-46-9View Details
Benzylamine 95% CAS 100-46-9View Details
100-46-9 -
 Benzylamine 98% CAS 100-46-9View Details
Benzylamine 98% CAS 100-46-9View Details
100-46-9 -
 Benzylamine CAS 100-46-9View Details
Benzylamine CAS 100-46-9View Details
100-46-9 -
 Benzylamine (SQ) CAS 100-46-9View Details
Benzylamine (SQ) CAS 100-46-9View Details
100-46-9 -
 Benzylamine CASView Details
Benzylamine CASView Details
