Pomalidomide
Synonym(s):1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline;3-amino-N-(2,6-dioxo-3-piperidyl)phthalamide;4-Amino-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;Actimid;IMiD 3
- CAS NO.:19171-19-8
- Empirical Formula: C13H11N3O4
- Molecular Weight: 273.24
- MDL number: MFCD12756407
- EINECS: 805-902-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
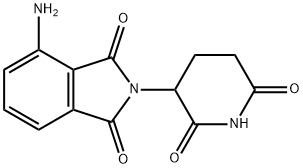
What is Pomalidomide?
Absorption
Pomalidomide is generally well absorbed. The major circulating component is the parent compound. Tmax, single oral dose = 2 -3 hours. When 4 mg of promalidomide is given to patients with multiple myeloma, the steady-state pharmacokinetic parameters are as follows: AUC(T) = 400 ng.hr/mL; Cmax = 75 ng/mL. Promalidomide accumulates following multiple doses.
Toxicity
Most common adverse reactions (≥30%) included fatigue and asthenia, neutropenia, anemia, constipation, nausea, diarrhea, dyspnea, upper-respiratory tract infections, back pain and pyrexia.
Description
In February 2013, the US FDA approved pomalidomide (also known as CC4047) for the treatment of multiple myeloma (MM) in patients with disease progression after receiving other cancer therapeutics. Pomalidomide is a 4-amino analog of thalidomide with enhanced potency and an improved toxicity profile. Pomalidomide and thalidomide exert their effects by modulation of immunity, inhibition of angiogenesis, interference with the bone/tumor microenvironment, and inhibition of the cereblon protein. Pomalidomide potently inhibited in vitro proliferation in a variety of human MM cell lines, IC50~10 nM, while thalidomide showed almost no inhibition up to 100 μM. In mouse MM tumor models, 50 mg/kg daily doses of pomalidomide resulted in marked inhibition of tumor growth after 15 days of treatment and complete regression in 3–6 weeks versus thalidomide-treated controls at the same dose. Pomalidomide is prepared by condensation of 4-nitrophthalic anhydride with 3-aminopiperidine-2,6-dione followed by catalytic hydrogenation of the nitro group.
Chemical properties
Yellow Solid
Originator
Celgene Corporation (United States)
The Uses of Pomalidomide
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM
The Uses of Pomalidomide
Pomalidomide is a thalidomide derivative, a potent inhibitor of TNF-α production. It is an antiinflammatory and antitumor agent used in the treatment of multiple myeloma.
The Uses of Pomalidomide
Pomalidomide is a second generation immunomodulator, TNF-α inhibitor, and thalidomide analog. An inhibitor of LPS-induced TNFαrelease.
Indications
Pomalidomide is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and have demonstrated disease progression on or within 60 days of completion of the last therapy. It is also indicated for the treatment of Kaposi's sarcoma (KS) in AIDS patients who have failed highly active antiretroviral therapy (HAART) and for the treatment of KS in HIV-negative patients.
Background
Pomalidomide, an analogue of thalidomide, is an immunomodulatory antineoplastic agent. FDA approved on February 8, 2013.
What are the applications of Application
Pomalidomide is an inhibitor of LPS-induced TNFαrelease
Definition
ChEBI: An aromatic amine that is thalidomide substituted at position 4 on the isoindole ring system by an amino group. Used for the treatment of multiple myeloma in patients who failed to respond to previous therapies.
brand name
Pomalyst
Biochem/physiol Actions
Pomalidomide is an effective fetal hemoglobin (HbF) inducer that downregulates the key γ-globin repressors, SRY-box transcription factor 6 (SOX6), and BAF chromatin remodeling complex subunit (BCL11A).
Pharmacokinetics
Pomalidomide is more potent than thalidomide (100-times) and lenalidomide (10-times).
Clinical Use
Treatment of multiple myeloma
Synthesis
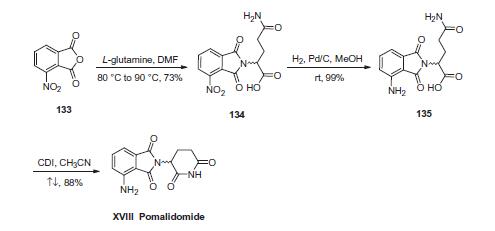
First, condensation of commercially available 3-nitrophthalic anhydride (133) and L-glutamine in warm DMF gave nitrophthalimide 134. Although the authors from Celgene do not explicitly describe the racemization of the stereocenter derived from L-glutamine, scrambling of the stereocenter has been reported during this step under neutral conditions at elevated temperatures. Next, hydrogenative reduction of the nitro group furnished the anilinophthalimide 135, and this was followed by treatment with CDI in refluxing acetonitrile to secure the piperidone dione and ultimately furnish pomalidomide (XVIII) as the racemate in 87% overall yield from 134.
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: concentration increased by
fluvoxamine.
Metabolism
Promalidomide is hepatically metabolized by CYP1A2 and CYP3A4. The metabolites are 26-fold less active than the parent compound. Minor contributions from CYP2C19 and CYP2D6 have been observed in vitro.
Metabolism
Mainly metabolised in the liver by the cytochrome P450
isoenzymes CYP1A2 and CYP3A4, with CYP2C19 and
CYP2D6 playing a minor role.
Following a single oral administration of
[14C]-pomalidomide (2 mg) to healthy subjects,
approximately 73% and 15% of the radioactive dose
was eliminated in urine and faeces, respectively, with
approximately 2% and 8% of the dosed radiocarbon
eliminated as pomalidomide in urine and faeces.
Storage
Store at +4°C
References
1) Lopez-Girona?et al.?(2012),?Cereblon is direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide; Leukemia,?26?2326 2) Zhu?et al.?(2013),?Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma; Leukemia Lymphoma,?54?683 3) Donovan?et al.?(2018),?Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome; Elife,?7?e38430 4) Winter?et al.?(2015),?DRUG DEVELOPMENT. Phthalimide conjunction as a strategy for in vivo target protein degradation; Science,?348?1376 5) Lohbeck and Miller (2016),?Practical synthesis of a phthalimide-based Cereblon ligand to enable PROTAC development; Bioorg. Med. Chem. Lett.,?26?5260
Properties of Pomalidomide
| Melting point: | 318.5 - 320.5° |
| Boiling point: | 582.9±45.0 °C(Predicted) |
| Density | 1.570±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥14mg/mL |
| form | powder |
| pka | 10.75±0.40(Predicted) |
| color | yellow |
| Merck | 14,135 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) |
Safety information for Pomalidomide
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Pomalidomide
| InChIKey | UVSMNLNDYGZFPF-UHFFFAOYSA-N |
| SMILES | C1(=O)C2=C(C(N)=CC=C2)C(=O)N1C1CCC(=O)NC1=O |
Pomalidomide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 19171-19-8 98%View Details
19171-19-8 98%View Details
19171-19-8 -
 Pomalidomide 98%View Details
Pomalidomide 98%View Details
19171-19-8 -
 19171-19-8 Pomalidomide 99%View Details
19171-19-8 Pomalidomide 99%View Details
19171-19-8 -
 19171-19-8 Pomalidomide 98%View Details
19171-19-8 Pomalidomide 98%View Details
19171-19-8 -
 Pomalidomide 98% CAS 19171-19-8View Details
Pomalidomide 98% CAS 19171-19-8View Details
19171-19-8 -
 Pomalidomide CAS 19171-19-8View Details
Pomalidomide CAS 19171-19-8View Details
19171-19-8 -
 Pomalidomide 99% (HPLC) CAS 19171-19-8View Details
Pomalidomide 99% (HPLC) CAS 19171-19-8View Details
19171-19-8 -
 Pomalidomide CAS 19171-19-8View Details
Pomalidomide CAS 19171-19-8View Details
19171-19-8
