Aniline
Synonym(s):Aminobenzene, Phenylamine, Benzenamine;Aniline
- CAS NO.:62-53-3
- Empirical Formula: C6H7N
- Molecular Weight: 93.13
- MDL number: MFCD00007629
- EINECS: 200-539-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
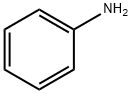
What is Aniline?
Description
Aniline—phenylamine or aminobenzene—smells like decaying fish and is a severe neurotoxin. But it’s also a very useful chemical building block, especially in dye chemistry. Aniline was isolated from indigo by O. Unverdorben in 1826 and from coal tar by F. Runge in 1834. C. J. Fritzsche synthesized and named it in 1841.?New uses for aniline include the preparation of flexible electrodes for supercapacitors from graphene–polyaniline composites.
Chemical properties
Aniline,C6H5NH2, is slightly soluble in water,miscible in alcohol and ether,and turns yellow to brown in air. Aniline is the end point of reduction of most mononitrogen substituted benzene nuclei,as nitro benzene beta-phenyl hydroxylamine, azoxybenzene, azobenzene, hydrazobenzene. Aniline is detected by the violet coloration produced by a small amountof sodium hypochlorite.
Physical properties
Colorless, oily liquid with a faint ammonia-like odor and burning taste. Gradually becomes yellow to reddish-brown on exposure to air or light. The lower and upper odor thresholds are 2 and 128 ppm, respectively (quoted, Keith and Walters, 1992). An odor threshold of 1.0 ppmv was reported by Leonardos et al. (1969).
The Uses of Aniline
Aniline is used as a solvent, in the preparation of compound in the manufacture of dyes and their intermediates, and in the manufacture of medicinal chemicals. Aniline is used in the manufacture of dyes,pharmaceuticals, varnishes, resins, photo graphic chemicals, perfumes, shoe blacks,herbicides, and fungicides. It is also usedin vulcanizing rubber and as a solvent. Itoccurs in coal tar and is produced from thedry distillation of indigo.
What are the applications of Application
Aniline is an oxidative stress, NF-κB and AP-1 inducer that has toxic effects on the spleen
Definition
ChEBI: Aniline is a primary arylamine in which an amino functional group is substituted for one of the benzene hydrogens. It is a primary arylamine and a member of anilines.
Production Methods
Aniline may be made(1) by the reduction, with iron or tin in HCI, of nitrobenzene, and(2) by the amination of chlorobenzene by heating with ammonia to a high temperature corresponding to a pressure of over 200 atmospheres in the presence of a catalyst(a mixture of cuprous chlorideandoxide).
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 29, p. 1159, 1981 DOI: 10.1248/cpb.29.1159
The Journal of Organic Chemistry, 58, p. 5620, 1993 DOI: 10.1021/jo00073a018
Air & Water Reactions
Darkens on exposure to air and light. Polymerizes slowly to a resinous mass on exposure to air and light. Slightly soluble in water.
Reactivity Profile
Aniline is a heat sensitive base. Combines with acids to form salts. Dissolves alkali metals or alkaline earth metals with evolution of hydrogen. Incompatible with albumin, solutions of iron, zinc and aluminum, and acids. Couples readily with phenols and aromatic amines. Easily acylated and alkylated. Corrosive to copper and copper alloys. Can react vigorously with oxidizing materials (including perchloric acid, fuming nitric acid, sodium peroxide and ozone). Reacts violently with BCl3. Mixtures with toluene diisocyanate may ignite. Undergoes explosive reactions with benzenediazonium-2-carboxylate, dibenzoyl peroxide, fluorine nitrate, nitrosyl perchlorate, peroxodisulfuric acid and tetranitromethane. Violent reactions may occur with peroxyformic acid, diisopropyl peroxydicarbonate, fluorine, trichloronitromethane (293° F), acetic anhydride, chlorosulfonic acid, hexachloromelamine, (HNO3 + N2O4 + H2SO4), (nitrobenzene + glycerin), oleum, (HCHO + HClO4), perchromates, K2O2, beta-propiolactone, AgClO4, Na2O2, H2SO4, trichloromelamine, acids, FO3Cl, diisopropyl peroxy-dicarbonate, n-haloimides and trichloronitromethane. Ignites on contact with sodium peroxide + water. Forms heat or shock sensitive explosive mixtures with anilinium chloride (detonates at 464° F/7.6 bar), nitromethane, hydrogen peroxide, 1-chloro-2,3-epoxypropane and peroxomonosulfuric acid. Reacts with perchloryl fluoride form explosive products.
Hazard
An allergen. Toxic if absorbed through the skin. Combustible. Skin irritant. Questionable car- cinogen.
Fire Hazard
Combustion can produce toxic fumes including nitrogen oxides and carbon monoxide. Aniline vapor forms explosive mixtures with air. Aniline is incompatible with strong oxidizers and strong acids and a number of other materials. Avoid heating. Hazardous polymerization may occur. Polymerizes to a resinous mass.
Flammability and Explosibility
Aniline is a combustible liquid (NFPA rating = 2). Smoke from a fire involving aniline may contain toxic nitrogen oxides and aniline vapor. Toxic aniline vapors are given off at high temperatures and form explosive mixtures in air. Carbon dioxide or dry chemical extinguishers should be used to fight aniline fires.
Biochem/physiol Actions
The acute toxicity of aniline involves its activation in vivo to 4-hydroxyaniline and the formation of adducts with hemoglobin. In erythrocytes, this is associated with the release of iron and the accumulation of methemoglobin and the development of hemolytic anemia and inflammation of the spleen. Tumor formation is often observed in the spleen on prolonged administration.
Safety Profile
Suspected carcinogen with experimental neoplastigenic data. A human poison by an unspecified route. Poison experimentally by most routes incluhng inhalation and ingestion. Experimental reproductive effects. A skin and severe eye irritant, and a rmld sensitizer. In the body, aniline causes formation of methemoglobin, resulting in prolonged anoxemia and depression of the central nervous system; less acute exposure causes hemolysis of the red blood cells, followed by stimulation of the bone marrow. The liver may be affected with resulting jaundice. Long-term exposure to a d n e dye manufacture has been associated with malignant bladder growths. A common air contaminant, A combustible liquid when exposed to heat or flame. To fight fire, use alcohol foam, CO2, dry chemical. It can react vigorously with oxidizing materials. When heated to decomposition it emits highly toxic fumes of NOx.
Potential Exposure
Aniline is widely used as an intermediate in the synthesis of dyestuffs. It is also used in the manufacture of rubber accelerators and antioxidants, pharmaceuticals, marking inks; tetryl, optical whitening agents; photographic developers; resins, varnishes, perfumes, shoe polishes, and many organic chemicals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
Carcinogenicity
The IARC has classified aniline as a Group 3 carcinogen, that is, not classifiable as to its carcinogenicity. However, NIOSH has determined that there is sufficient evidence to recommend that OSHA require labeling this substance a potential occupational carcinogen. This position followed an evaluation of a high-dose feeding study of aniline hydrochloride in F344 rats and B6C3F1 mice (3000 or 6000 ppm and 6000 or 12,000 ppm, respectively). The test was negative in both sexes of mice; however, hemangiosarcomas of the spleen and combined incidence of fibrosarcomas and sarcomas of the spleen were statistically significant in the male rats; the number of female rats having fibrosarcomas of the spleen was also significant.
Source
Detected in distilled water-soluble fractions of regular gasoline (87 octane) and Gasohol
at concentrations of 0.55 and 0.20 mg/L, respectively (Potter, 1996). Aniline was also detected in
82% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from Boston, MA). At
25 °C, concentrations ranged from 70 to 16,000 μg/L in gasoline and 20 to 3,800 μg/L in watersoluble
fractions. Average concentrations were 5.8 mg/L in gasoline and 1.4 mg/L in watersoluble
fractions (Schmidt et al., 2002).
Based on laboratory analysis of 7 coal tar samples, aniline concentrations ranged from ND to 13
ppm (EPRI, 1990).
Aniline in the environment may originate from the anaerobic biodegradation of nitrobenzene
(Razo-Flores et al., 1999).
Storage
Before entering confined space where aniline may be present, check to make sure that an explosive concentration doesnot exist. Store in tightly closed containers in a cool, dry,dark, well-ventilated area. Metal containers involving thetransfer of this chemical should be grounded and bonded.Where possible, automatically pump liquid from drums orother storage containers to process containers. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters.
Shipping
Aninline requires a shipping label of“POISONOUS/TOXIC MATERIALS.” It falls in HazardClass 6.1 and the Packing Group is II.[19, 20] Aniline carriesa plus sign ( 1 ), indicating that the designated proper shipping name and hazard class of the material must always beshown whether or not the material or its mixtures or solutions meet the definitions of the class. The hydrochloriderequires a “POISONOUS/TOXIC MATERIALS” label. Itfalls in Hazard Class 6.1 and Packing Group III.
Incompatibilities
May form explosive mixture with air. Unless inhibited (usually methanol), aniline is readily able to polymerize. Fires and explosions may result from contact with halogens, strong acids; oxidizers, strong base organic anhydrides; acetic anhydride, isocyanates, aldehydes, sodium peroxide. Strong reaction with toluene diisocyanate. Reacts with alkali metals and alkali earth metals. Attacks some plastics, rubber and coatings; copper and copper alloys.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with provision for nitrogen oxides removal from flue gases by scrubber, catalytic or thermal device.
Properties of Aniline
| Melting point: | -6 °C (lit.) |
| Boiling point: | 184 °C (lit.) |
| Density | 1.022 g/mL at 25 °C (lit.) |
| vapor density | 3.22 (185 °C, vs air) |
| vapor pressure | 0.7 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 76 °C |
| storage temp. | 2-8°C |
| solubility | water: soluble |
| form | Liquid |
| pka | 4.63(at 25℃) |
| Specific Gravity | 1.021 |
| color | APHA: ≤250 |
| Odor | Sweet, amine-like odor detectable at 0.6 to 10 ppm |
| PH Range | 8.1 |
| Relative polarity | 0.42 |
| PH | 8.8 (36g/l, H2O, 20℃) |
| explosive limit | 1.2-11%(V) |
| Water Solubility | 36 g/L (20 ºC) |
| Merck | 14,659 |
| BRN | 605631 |
| Henry's Law Constant | 1.91 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Dielectric constant | 7.8(0℃) |
| Exposure limits | TLV-TWA skin 2 ppm (~8 mg/m3) (ACGIH),
5 ppm (~19 mg/m3) (MSHA, OSHA, and
NIOSH); IDLH 100 ppm (NIOSH). |
| Stability: | Stable. Incompatible with oxidizing agents, bases, acids, iron and iron salts, zinc, aluminium. Light sensitive. Combustible. |
| CAS DataBase Reference | 62-53-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Aniline(62-53-3) |
| IARC | 2A (Vol. 27, Sup 7, 127) |
| EPA Substance Registry System | Aniline (62-53-3) |
Safety information for Aniline
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aniline
Aniline manufacturer
Crystal Chemitrade
Paragon Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Aniline Oil 99%View Details
Aniline Oil 99%View Details -
 Aniline CAS 62-53-3View Details
Aniline CAS 62-53-3View Details
62-53-3 -
 Aniline CAS 62-53-3View Details
Aniline CAS 62-53-3View Details
62-53-3 -
 Aniline CAS 62-53-3View Details
Aniline CAS 62-53-3View Details
62-53-3 -
 Aniline Oil CASView Details
Aniline Oil CASView Details -
 Aniline Oil CASView Details
Aniline Oil CASView Details -
 Liquid Aniline Oil Fresh, Grade Standard: Technical Grade, Packaging Type: DrumView Details
Liquid Aniline Oil Fresh, Grade Standard: Technical Grade, Packaging Type: DrumView Details
62-53-3 -
 Liquid ANILINE 99% Extra Pure, Packaging Size: 2.5 LView Details
Liquid ANILINE 99% Extra Pure, Packaging Size: 2.5 LView Details
62-53-3
