Malonamide
Synonym(s):Malonodiamide
- CAS NO.:108-13-4
- Empirical Formula: C3H6N2O2
- Molecular Weight: 102.09
- MDL number: MFCD00008034
- EINECS: 203-553-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
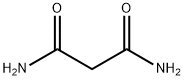
What is Malonamide?
Description
Malonamide is a dicarboxylic acid diamide that is malonic acid in which both carboxy groups have been replaced by carbamoyl groups. It is functionally related to a malonic acid.
Chemical properties
White crystalline powder
The Uses of Malonamide
Malonamide is used in the synthesis of malonamic acid, malonamate and malonamide derivative of some heterocyclic compounds with antiinflammatory activity.
The Uses of Malonamide
Malonamide is a reagent for fluorimetric determination of reducing carbohydrates. The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare earth elements from nitric acid medium.
The Uses of Malonamide
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
What are the applications of Application
Malonamide is a reagent for fluorimetric determination of reducing carbohydrates
Definition
ChEBI: Malonamide is a dicarboxylic acid diamide that is malonic acid in which both carboxy groups have been replaced by carbamoyl groups. It is functionally related to a malonic acid.
General Description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Hazard
Mildly toxic by ingestion.
Synthesis
The synthesis of Malonamide is as follows:
A typical reaction was carried out in a 10 mL flask. Benzonitrile (2 mmol), CuII-4 ? (0.2 g), acetaldoxime (6 mmol) and MeOH (4 mL) were stirred at 65 °C for 4 h. The solid was filtered, washed with MeOH and the filtrate evaporated. The residue was subjected to GC-MS analysis and NMR spectroscopy. The filtered catalyst can be recycled after drying at about 150 °C for 1 h.
Purification Methods
Crystallise the amide from water. [Beilstein 2 IV 1887.]
Properties of Malonamide
| Melting point: | 172-175 °C (lit.) |
| Boiling point: | 191.38°C (rough estimate) |
| Density | 1.3516 (rough estimate) |
| refractive index | 1.5110 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in HCl. |
| form | powder to crystal |
| pka | 7.00±0.70(Predicted) |
| color | White to Almost white |
| Water Solubility | 180 g/L (20 ºC) |
| BRN | 1751401 |
| InChI | InChI=1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7) |
| CAS DataBase Reference | 108-13-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanediamide(108-13-4) |
| EPA Substance Registry System | Propanediamide (108-13-4) |
Safety information for Malonamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Malonamide
| InChIKey | WRIRWRKPLXCTFD-UHFFFAOYSA-N |
| SMILES | C(N)(=O)CC(N)=O |
Malonamide manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 108-13-4 MALONODIAMIDE 98%View Details
108-13-4 MALONODIAMIDE 98%View Details
108-13-4 -
 Malonamide CAS 108-13-4View Details
Malonamide CAS 108-13-4View Details
108-13-4 -
 Malonodiamide 98.00% CAS 108-13-4View Details
Malonodiamide 98.00% CAS 108-13-4View Details
108-13-4 -
 Malonamide 98% CAS 108-13-4View Details
Malonamide 98% CAS 108-13-4View Details
108-13-4 -
 Malonamide CAS 108-13-4View Details
Malonamide CAS 108-13-4View Details
108-13-4 -
 Malonamide CAS 108-13-4View Details
Malonamide CAS 108-13-4View Details
108-13-4 -
 Malonamide extrapure CAS 108-13-4View Details
Malonamide extrapure CAS 108-13-4View Details
108-13-4 -
 Malonamide CAS 108-13-4View Details
Malonamide CAS 108-13-4View Details
108-13-4