Axitinib
Synonym(s):AG-013736;N-Methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide
- CAS NO.:319460-85-0
- Empirical Formula: C22H18N4OS
- Molecular Weight: 386.47
- MDL number: MFCD09837898
- EINECS: 638-771-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Axitinib?
Absorption
After one 5 mg dose of axitinib, it takes about 2.5 to 4.1 hours to reach maximum plasma concentration.
Toxicity
Some of the more serious toxic effects seen in patients taking axitinib include, but are not limited to, hypertension, thrombotic events, hemorrhage, and GI perforation.
Description
Axitinib is a VEGFR inhibitor (IC50s = 1.2, 0.25, and 0.29 nM for VEGFR1, -2, and -3, respectively). It also inhibits c-Kit and PDGFRβ (IC50s = 1.7 and 1.6 nM, respectively). It inhibits VEGF-induced migration of and tube formation by human umbilical vein endothelial cells (HUVECs). In January 2012, the US FDA approved axitinib (also referred to as AG-013736) for the treatment of advanced renal cell carcinoma (RCC) for patients who have not responded to prior therapy.
Chemical properties
Off-White Solid
Characteristics
Class: receptor tyrosine kinase
Treatment: RCC
Oral bioavailability = 58%
Elimination half-life = 2.5–6.1 h
Protein binding = 99%
The Uses of Axitinib
Axitinib (AG-013736) is a multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ and c-Kit with IC50 of 0.1 nM, 0.2 nM, 0.1-0.3 nM, 1.6 nM and 1.7 nM, respectively. Through this mechanism of action, axitinib blocks angiogenesis, tumour growth and metastases. It is reported to exhibit potency that is 50-450 times higher than that of the first generation VEGFR inhibitors. Axitinib is an indazole derivative.
Indications
Used in kidney cell cancer and investigated for use/treatment in pancreatic and thyroid cancer.
Definition
ChEBI: An indazole substituted at position 3 by a 2-(pyridin-2-yl)vinyl group and at position 6 by a 2-(N-methylaminocarboxy)phenylsulfanyl group. Used for the treatment of advanced renal cell carcinoma after failure of a first line systemic tr atment.
Biochem/physiol Actions
Axitinib (AG-013736) is an orally available, potent (picomolar) and selective tyrosine kinase inhibitor that blocks VEGF receptors 1, 2 and 3. The drug blocks VEGF-mediated endothelial cell survival, tube formation, and downstream signaling through endothelial nitric oxide synthase, Akt and extracellular signal-regulated kinase.
Pharmacokinetics
Axitinib prevents the progression of cancer by inhibiting angiogenesis and blocking tumor growth.
Clinical Use
Sold under the brand name Inlyta® by Pfizer, Inc., axitinib was approved by the FDA in January 2012 for the treatment of advanced renal cell carcinoma (RCC), specifically after the failure of other systemic treatments. Axitinib slows cancer cell proliferation by inhibition of the vascular endothelial growth factor (VEGF)/VEGF receptor tyrosine (RTK) signaling pathway. In particular, axitinib is a potent inhibitor of VEGF/RTK 1-3, which selectively slows angiogenesis, vascular permeability, and blood flow in solid tumors.
Side Effects
The side effects that you should report to your doctor include:
allergic reactions like skin rash, itching or hives, swelling of the face, lips, or tongue;
high blood pressure;
seizures;
signs and symptoms of bleeding such as bloody or black, tarry stools; red or dark-brown urine; spitting up blood or brown material that looks like coffee grounds; red spots on the skin; unusual bruising or bleeding from the eye, gums, or nose;
signs and symptoms of a blood clot such as breathing problems; changes in vision; chest pain; severe, sudden headache; pain, swelling, warmth in the leg; trouble speaking; sudden numbness or weakness of the face, arm, or leg;
stomach pain.
The side effects that usually do not require medical attention include constipation cough, diarrhea, loss of appetite, nausea, and vomiting.
Synthesis
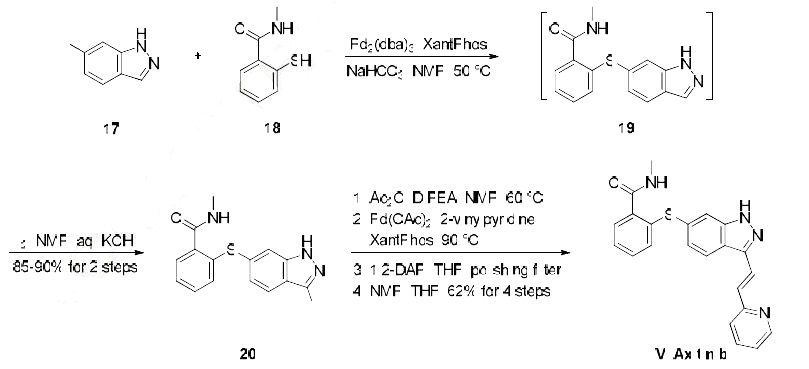
Numerous patents and papers have been disclosed on the synthesis of axitinib, a recently published manuscript details the development of the manufacturing route, and this route is depicted in the scheme. The synthesis began with Migita coupling of commercial iodide 17 with thiophenol 18. Interestingly, this transformation?ˉs efficiency relied upon attention to the number of equivalents of base and an inert atmosphere in the reaction vessel, conditions which minimized catalyst poisoning during the reaction. Without isolation, indazole 19 was iodinated to afford diarylthioether 20 in 85-90% yield over the two steps. Protection of the indazole within 20 as its acetamide preceeded a Heck reaction with 2-vinylpyridine, and then subsequent removal of the indazole protection followed by a series of recrystallizations yielded axitinib (IV) in a combined 62% yield over the final 4 steps.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis); avoid with pimozide.
Concomitant use with strong CYP3A4/5 inhibitors:
avoid; however, if concomitant use cannot be avoided
then reduce the dose of axitinib by approximately
half; subsequent doses can be increased or decreased
based on individual safety and tolerability; if
CYP3A4/5 inhibitor is discontinued, then increase
the axitinib dose used prior to initiation of the
strong inhibitor after 3-5 half-lives of the inhibitor
(strong CYP3A4/5 inhibitors include ketoconazole,
itraconazole, clarithromycin, atazanavir, indinavir,
ritonavir, saquinavir, and voriconazole).
Metabolism
Axitinib undergoes mainly hepatic metabolism. CYP3A4 and CYP3A5 are the main hepatic enzymes while CYP1A2, CYP2C19, and UGT1A1 enzymes are secondary.
References
1) Hu-Lowe?et al.?(2008),?Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1,2,3; Cancer Res.,?14?7272 2) Ma and Waxman (2008),?Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib; Mol. Cancer Ther.,?7?79 3) Pemovska?et al.?(2015),?Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation; Nature,?519?102 4) Rixe?et al.?(2007),?Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer; a phase II study; Lancet Oncol.,?8?975 5) Yuan?et al.?(2014),?Axitinib augments antitumor activity in renal cell carcinoma via STAT3-dependent reversal of myeloid-derived suppressor cell accumulation;?Biomed.Pharmacother.?68?751 6) Zhang?et al.?(2014),?Axitinib, a selective inhibitor of vascular endothelial growth factor receptor, exerts an anticancer effect in melanoma through promoting antitumor immunity;?Anticancer Drugs?25?204
Properties of Axitinib
| Melting point: | 213-215°C |
| Boiling point: | 668.9±55.0 °C(Predicted) |
| Density | 1.4 |
| storage temp. | room temp |
| solubility | DMSO: ≥8mg/mL |
| form | powder |
| pka | 12.70±0.40(Predicted) |
| color | white to tan |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 319460-85-0(CAS DataBase Reference) |
Safety information for Axitinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Axitinib
| InChIKey | RITAVMQDGBJQJZ-FMIVXFBMSA-N |
Axitinib manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Axitinib 98%View Details
Axitinib 98%View Details
319460-85-0 -
 AXITINIB 95-99 %View Details
AXITINIB 95-99 %View Details
319460-85-0 -
 Axitinib 98% CAS 319460-85-0View Details
Axitinib 98% CAS 319460-85-0View Details
319460-85-0 -
 Axitinib IHSView Details
Axitinib IHSView Details
319460-85-0 -
 Axitinib Api PowderView Details
Axitinib Api PowderView Details
319460-85-0 -
 Axitinib 5mg Tablets - Anti-Cancer Medicines Exporter from India - Bulk SupplyView Details
Axitinib 5mg Tablets - Anti-Cancer Medicines Exporter from India - Bulk SupplyView Details
319460-85-0 -
 Axitinib Powder APIView Details
Axitinib Powder APIView Details
319460-85-0 -
 Axitinib 5mg TabletsView Details
Axitinib 5mg TabletsView Details
319460-85-0