Bosutinib
Synonym(s):4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile;SKI-606;WAY-173606
- CAS NO.:380843-75-4
- Empirical Formula: C26H29Cl2N5O3
- Molecular Weight: 530.45
- MDL number: MFCD07367846
- EINECS: 700-455-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
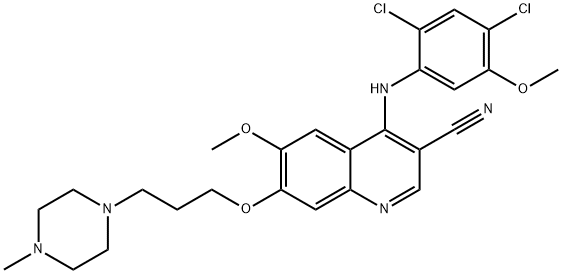
What is Bosutinib?
Absorption
Bosutinib exhibits dose-proportional increases in Cmax and AUC over the oral dose range of 200 to 800 mg (0.33 to 1.3 times the maximum approved recommended dosage of 600 mg). Bosutinib steady-state Cmax was 127 ng/mL (31%), Ctrough was 68 ng/mL (39%) and AUC was 2370 ng?h/mL (34%) following multiple oral doses of bosutinib 400 mg. Bosutinib steady-state Cmax was 171 ng/mL (38%), Ctrough was 91 ng/mL (42%) and AUC was 3150 ng?h/mL (38%) following multiple oral doses of bosutinib 500 mg. No clinically significant differences in the pharmacokinetics of bosutinib were observed following administration of either the tablet or capsule dosage forms of bosutinib at the same dose, under fed conditions.
The median bosutinib (minimum, maximum) tmax was 6.0 (6.0, 6.0) hours following oral administration of a single oral dose of bosutinib 500 mg with food. The absolute bioavailability was 34% in healthy subjects.
Bosutinib Cmax increased 1.8-fold and AUC increased 1.7-fold when bosutinib tablets were given with a high-fat meal to healthy subjects compared to administration under fasted conditions. Bosutinib Cmax increased 1.6-fold and AUC increased 1.5-fold when bosutinib capsules were given with a high-fat meal to healthy subjects compared to administration under fasted conditions. The high-fat meal (800-1000 total calories) consisted of approximately 150 protein calories, 250 carbohydrate calories, and 500-600 fat calories.
Toxicity
In a rat fertility and early embryonic development study, bosutinib was administered orally to female rats for approximately 3 to 6 weeks, depending on the day of mating (2 weeks prior to cohabitation with untreated breeder males until gestation day [GD] 7). Increased embryonic resorptions occurred at greater than or equal to 10 mg/kg/day of bosutinib (1.6 and 1.2 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively), and decreased implantations and reduced number of viable embryos at 30 mg/kg/day of bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
In an embryo-fetal development study conducted in rabbits, bosutinib was administered orally to pregnant animals during organogenesis at doses of 3, 10, and 30 mg/kg/day. At the maternally-toxic dose of 30 mg/kg/day of bosutinib, there were fetal anomalies (fused sternebrae and 2 fetuses had various visceral observations), and an approximate 6% decrease in fetal body weight. The dose of 30 mg/kg/day resulted in exposures (AUC) approximately 5.1 and 3.8 times the human exposures at the recommended doses of 400 and 500 mg/day, respectively.
Fetal exposure to bosutinib-derived radioactivity during pregnancy was demonstrated in a placental-transfer study in pregnant rats. In a rat pre-and postnatal development study, bosutinib was administered orally to pregnant animals during the period of organogenesis through lactation day 20 at doses of 10, 30, and 70 mg/kg/day. Reduced number of pups born occurred at greater than or equal to 30 mg/kg/day bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively), and increased incidence of total litter loss and decreased growth of offspring after birth occurred at 70 mg/kg/day bosutinib (6.9 and 5.1 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
Experience with bosutinib overdose in clinical studies was limited to isolated cases. There were no reports of any serious adverse events associated with the overdoses. Patients who take an overdose of BOSULIF should be observed and given appropriate supportive treatment.
Bosutinib was not carcinogenic in rats or transgenic mice. The rat 2-year carcinogenicity study was conducted at bosutinib oral doses up to 25 mg/kg in males and 15 mg/kg in females. Exposures at these doses were approximately 1.5 times (males) and 3.1 times (females) the human exposure at the 400 mg dose and 1.2 times (males) and 2.4 times (females) exposure in humans at the 500 mg dose. The 6-month RasH2 transgenic mouse carcinogenicity study was conducted at bosutinib oral doses up to 60 mg/kg.
Bosutinib was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames Test), the in vitro assay using human peripheral blood lymphocytes and the micronucleus test in orally treated male mice.
In a rat fertility study, drug-treated males were mated with untreated females or untreated males were mated with drug-treated females. Females were administered the drug from pre-mating through early embryonic development. The dose of 70 mg/kg/day of bosutinib resulted in reduced fertility in males as demonstrated by 16% reduction in the number of pregnancies. There were no lesions in the male reproductive organs at this dose. This dose of 70 mg/kg/day resulted in exposure (AUC) in male rats approximately 1.5 times and equal to human exposure at the recommended doses of 400 and 500 mg/day, respectively. Fertility (number of pregnancies) was not affected when female rats were treated with bosutinib. However, there were increased embryonic resorptions at greater than or equal to 10 mg/kg/day of bosutinib (1.6 and 1.2 times the human exposure at the recommended doses of 400 and 500 mg/day, respectively), and decreased implantations and reduced number of viable embryos at 30 mg/kg/day of bosutinib (3.4 and 2.5 times the human exposure at the recommended doses of 400 or 500 mg/day, respectively).
Description
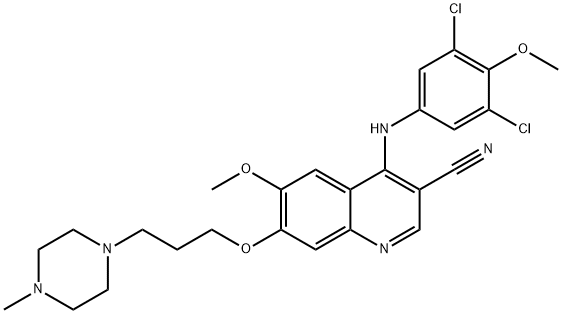
Bosutinib is a tyrosine kinase inhibitor being developed by Pfizer to treat chronic myeloid leukemia. During the Phase III trials, an unusual problem occurred. N. M. Levinson at Stanford University noticed that a?databank structure of bosutinib bound to a kinase?was inconsistent with authentic bosutinib. It turned out that a methoxy group and two chlorine atoms on the molecule’s aniline substituent were in the wrong positions (see lower inset). An investigation showed that the “wrong” isomer has been used in many medical trials since 2006.
Description
In September 2012, the US FDA approved bosutinib (also referred to as SKI-607) for the treatment of relapsed or refractory chronicmyeloid leukemia (CML) for patients with resistance or intolerance to prior therapy. The National Cancer Institute estimates that in 2012, 5430 men and women were diagnosed with CML and 610 died of CML. First and second-line therapies for the treatment of CML include imatinib, dasatinib, and nilotinib. Bosutinib, which was initially identified as a Src inhibitor, was later found to be a dual inhibitor of Bcr–Abl (IC50=1.4 nM) and Src family kinases (IC50=3.5 nM). Bosutinib inhibits 16 of 18 imatinib-resistant forms of Bcr–Abl expressed in murine myeloid cell lines. In preclinical in vivo studies, bosutinib at 15 mg/kg administered orally for 5 days caused regression of K562 CML tumors in nude mice and in BaF3 tumors expressing wild type or different imatinib-resistant Bcr–Abl mutants at varying doses. A manufacturing process for the synthesis of bosutinib monohydrate has been reported that employs a key three-component cyclization reaction involving an aniline, a cyanoacetamide intermediate, and triethyl orthoformate followed by cyclization using phosphorous oxytrichloride and an optimized hydration procedure.
Chemical properties
Pale Yellow Solid
Class: non-receptor tyrosine kinase
Treatment: CML
Oral bioavailability = 34%
Elimination half-life = 22.5 h
Protein binding = 95%
Originator
Wyeth (United States)
The Uses of Bosutinib
Bosutinib (SKI-606) is a novel, dual Src/Abl inhibitor with IC50 of 1.2 nM and 1 nM, respectively.
The Uses of Bosutinib
A novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells.
Background
Bosutinib is a 7-alkoxy-3-quinolinecarbonitrile that functions as a potent, dual SRC and ABL tyrosine kinase inhibitor indicated for chronic myelogenous leukemia (CML), specifically Philadelphia chromosome-positive (Ph+) CML. Philadelphia chromosome is a hallmark of CML due to the reciprocal translocation t(9;22)(q34;q11), resulting in a BCR-ABL fusion protein. The first BCR-ABL inhibitor, imatinib, was introduced over a decade ago as a breakthrough in CML management; however, emerging resistance to imatinib poses challenges in achieving remission. Second-generation BCR-ABL inhibitors like bosutinib inhibit most resistance-conferring BCR-ABL mutations except V299L and T315, thus providing more therapeutic options for patients.
Bosutinib was first approved by the FDA in 2012 for the treatment of adult chronic, accelerated, or blast-phase Ph+ CML with resistance or intolerance to prior therapy. On September 26, 2023, bosutinib was also approved by the FDA for the treatment of pediatric CML that is newly diagnosed or resistant/intolerant to prior therapy. This approval was based on favorable results obtained from the open-label, randomized, multicenter trial BFORE that showed a significant improvement in major molecular response, defined as a ≤0.1% BCR ABL ratio on an international scale, with bosutinib treatment.
Indications
Bosutinib is indicated for the treatment of adult and pediatric patients 1 year of age and older with chronic phase Philadelphia chromosome-positive chronic myelogenous leukemia that is newly diagnosed or resistant or intolerant to prior therapy. It is also indicated for the treatment of adult patients with accelerated or blast phase Philadelphia chromosome-positive chronic myelogenous leukemia that is newly diagnosed or resistant or intolerant to prior therapy.
Definition
ChEBI: Bosutinib is an aminoquinoline that is 4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline bearing additional cyano and methoxy substituents at positions 3 and 6 respectively. It has a role as an antineoplastic agent and a tyrosine kinase inhibitor. It is a nitrile, a N-methylpiperazine, an aromatic ether, a tertiary amino compound, an aminoquinoline and a dichlorobenzene.
brand name
Bosulif
General Description
Bosutinib is a safe oral medicine with good bioavailability. It is effective for treating chronic myeloid leukemia (CML). It is also a potent ATP-competitive inhibitor for Src tyrosine kinase. Bosutinib inhibits epidermal growth factor receptor (EGFR). It suppresses solid tumors associated with breast, pancreas and prostate.
Biochem/physiol Actions
Bosutinib (SKI-606) is an orally active; dual Src/Abl tyrosine kinase inhibitor with potent antiproliferative activity. It does not appear to inhibit c-Kit and PDGRF, which are thought to be the cause of numerous side effects in anticancer treatment with some other tyrosine kinase inhibitors.
Pharmacokinetics
A greater likelihood of response and a greater likelihood of safety events were observed with higher bosutinib exposure in clinical studies. The time course of bosutinib pharmacodynamic response has not been fully characterized.
At a single oral dose of 500 mg bosutinib with ketoconazole (a strong CYP3A inhibitor), bosutinib does not prolong the QT interval to any clinically relevant extent.
Clinical Use
Protein kinase inhibitor:
Treatment of Philadelphia chromosome-positive
chronic myelogenous leukaemia resistant or
intolerant to prior therapy
Side Effects
Possible side effects of bosutinib include Nausea, vomiting, stomach/abdominal pain, loss of appetite, cough, joint pain, headache, or dizziness may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly. Diarrhea is a common side effect.
www.webmd.com
Drug interactions
Potentially hazardous interactions with other drugs Analgesics: possibly increased risk of ventricular arrhythmias with methadone. Anti-arrhythmics: possibly increased risk of ventricular arrhythmias with amiodarone and disopyramide; concentration possibly increased by dronedarone - avoid or consider reducing dose of bosutinib. Antibacterials: concentration possibly increased by ciprofloxacin, clarithromycin, erythromycin and telithromycin - avoid or consider reducing dose of bosutinib; possibly increased risk of ventricular arrhythmias with moxifloxacin; concentration reduced by rifampicin and possibly rifabutin - avoid. Antidepressants: concentration possibly reduced by St John’s wort - avoid. Antiepileptics: concentration possibly reduced by carbamazepine, fosphenytoin, phenobarbital, phenytoin and primidone - avoid. Antifungals: concentration increased by ketoconazole and possibly by fluconazole, itraconazole, posaconazole and voriconazole - avoid or consider reducing dose of bosutinib. Antimalarials: possibly increased risk of ventricular arrhythmias with chloroquine and hydroxychloroquine. Antipsychotics: possibly increased risk of ventricular arrhythmias with haloperidol; avoid with clozapine, increased risk of agranulocytosis. Antivirals: concentration possibly increased by atazanavir, boceprevir, darunavir, fosamprenavir, indinavir, ritonavir, saquinavir and telaprevir - avoid or consider reducing dose of bosutinib; concentration possibly reduced by efavirenz and etravirine - avoid. Aprepitant: concentration possibly increased - avoid or consider reducing dose of bosutinib. Beta-blockers: possibly increased risk of ventricular arrhythmias with sotalol. Bosentan: concentration of bosutinib possibly reduced - avoid. Calcium channel blockers: concentration possibly increased by diltiazem or verapamil - avoid or consider reducing dose of bosutinib. Cytotoxics: concentration possibly increased by imatinib - avoid or consider reducing dose of bosutinib. Domperidone: avoid concomitant use, risk of ventricular arrhythmias. Fosaprepitant: concentration possibly increased by fosaprepitant - avoid or consider reducing dose of bosutinib Grapefruit juice: concentration possibly increased by grapefruit juice - avoid or consider reducing dose of bosutinib. Modafinil: concentration of bosutinib possibly reduced - avoid.
Metabolism
Bosutinib is primarily metabolized by CYP3A4. The major circulating metabolites identified in plasma are oxydechlorinated (M2) bosutinib (19% of parent exposure) and N-desmethylated (M5) bosutinib (25% of parent exposure), with bosutinib N-oxide (M6) as a minor circulating metabolite. All the metabolites were deemed inactive.
Metabolism
Mainly hepatically metabolised. The major circulating metabolites identified in plasma are oxydechlorinated (M2) bosutinib (19% of parent exposure) and N-desmethylated (M5) bosutinib (25% of parent exposure), with bosutinib N-oxide (M6) as a minor circulating metabolite. All the metabolites are inactive. Excretion is mainly via the faeces.
Storage
+4°C
Mode of action
Bosutinib is a synthetic quinolone derivative and dual kinase inhibitor that targets both Abl and Src kinases with potential antineoplastic activity. Unlike imatinib, bosutinib inhibits the autophosphorylation of both Abl and Src kinases, resulting in inhibition of cell growth and apoptosis. Because of the dual mechanism of action, this agent may have activity in resistant CML disease, other myeloid malignancies and solid tumors. Abl kinase is upregulated in the presence of the abnormal Bcr-abl fusion protein which is commonly associated with chronic myeloid leukemia (CML). Overexpression of specific Src kinases is also associated with the imatinib-resistant CML phenotype.
References
Golas et al. (2003), SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice; Cancer Res., 63 375 Remsing Rix et al. (2009), Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells; Leukemia, 23 477 Redaelli et al. (2009), Activity of bosutinib, Dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants; J. Clin. Oncol., 27 469 MacDonald et al. (2018), Src family kinase inhibitor bosutinib enhances retinoic acid-induced differentiation of HL-60 leukemia cells; Leuk. Lymphoma, 59 2941 Vultur et al. (2008), SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells; Mol. Cancer Ther., 7 1185
Properties of Bosutinib
| Melting point: | 116-120 ºC |
| Boiling point: | 649.7±55.0 °C(Predicted) |
| Density | 1.36 |
| vapor pressure | 0-0Pa at 20℃ |
| storage temp. | room temp |
| solubility | DMSO: ≥15mg/mL |
| form | powder |
| pka | 7.63±0.10(Predicted) |
| color | off-white to light brown |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 380843-75-4(CAS DataBase Reference) |
Safety information for Bosutinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bosutinib
| InChIKey | PICKMQXYAMDEPF-UHFFFAOYSA-N |
Bosutinib manufacturer
Aquigen Bio science Pvt Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![3-Quinolinecarbonitrile,4-chloro-6-Methoxy-7-[3-(4-Methyl-1-piperazinyl)propoxy]-](https://img.chemicalbook.in/CAS/20150408/GIF/492444-39-0.gif)

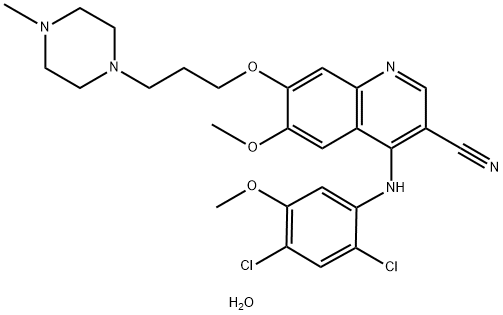
You may like
-
 380843-75-4 Bosutinib 98%View Details
380843-75-4 Bosutinib 98%View Details
380843-75-4 -
 380843-75-4 99%View Details
380843-75-4 99%View Details
380843-75-4 -
 380843-75-4 BOSUTINIB 95-99%View Details
380843-75-4 BOSUTINIB 95-99%View Details
380843-75-4 -
 Bosutinib 95% CAS 380843-75-4View Details
Bosutinib 95% CAS 380843-75-4View Details
380843-75-4 -
 Bosutinib, ≥98% (HPLC) CAS 380843-75-4View Details
Bosutinib, ≥98% (HPLC) CAS 380843-75-4View Details
380843-75-4 -
 380843-75-4 Bosutinib 98%View Details
380843-75-4 Bosutinib 98%View Details
380843-75-4 -
 Blood Cancer Bosutinib 400 Mg 30 Tab , 500mg 30 Tab , 100mg 60 Tab Bosutris MylanView Details
Blood Cancer Bosutinib 400 Mg 30 Tab , 500mg 30 Tab , 100mg 60 Tab Bosutris MylanView Details
380843-75-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
